This study is an identification of polymorphisms in genes associated with hereditary thrombophilia and other disorders of the blood coagulation system. Thrombophilia is an increased tendency to random (for no apparent reason) deep vein thrombosis and thromboembolic complications. Thrombosis can occur due to circulatory disorders (blood stagnation), increased ability of the blood to form blood clots (hypercoagulation), damage to the vascular wall, and a combination of these factors. Hypercoagulation may be due to the presence of a genetic predisposition to thrombophilia (the most common polymorphisms in the F2 and F5 genes), which this analysis can identify.
When do you need to take the THROMBOPHILIA extended test?
- Cases of hereditary thromboembolism in the family.
- History of thrombosis:
- single thrombosis before 50 years of age;
- repeated thromboses;
- a case of thrombosis at any age with a family history;
- thrombosis of unusual localization (portal, mesenteric, cerebral veins);
- thrombosis of unknown etiology after 50 years.
- High risk situations:
- massive surgical interventions;
- long-term immobilization;
- oncological diseases;
- chemotherapy.
- Ischemic stroke.
- Cardiovascular diseases.
- Prescription of standard antiplatelet therapy.
- Use of hormonal contraception or hormone replacement therapy.
- Planning pregnancy in women with a history of thrombosis, 1st degree relatives with diagnosed hereditary thrombophilia, or a family history of thromboembolic complications.
- Complicated obstetric history.
What do the test results mean?
As a conclusion, a genetic health card is issued, compiled by a professional geneticist in our laboratory. It presents the results of the analysis with interpretation, provides a detailed description of the studied polymorphisms and their impact on the risks of developing disorders of the blood coagulation system, cardiovascular diseases and pathologies of pregnancy, advice on conducting additional studies and detailed clinical recommendations for the attending physician.
The results of the analysis are interpreted by the attending physician. Based on them, the doctor can adjust the patient’s diet and lifestyle, prescribe medications and additional tests.
Detailed description of the study
A study of 12 genetic factors in the development of thrombophilia is used to assess the risk of thrombosis and other complications in people at risk.
The study includes analysis of 12 parameters:
- Coagulation factor II (F2 thrombin). Polymorphism: G20210A.
- Coagulation factor V (F5 Factor Leiden) Factor V Leiden (G1691A; Arg506Gln).
- Coagulation factor VII (proconvertin) F7: 10976 G>A (Arg353Gln).
- Coagulation factor XIII (F13A1). Polymorphism: Val34Leu (Val35Leu).
- Fibrinogen (Coagulation factor 1) FGB: G-455A (G-467A).
- Integrin, alpha 2 ITGA2: C807T.
- Integrin, beta 3 (platelet glycoprotein IIIa) ITGB3: PIA1/PIA2 (Leu33Pro; T1565C; HPA-1b).
- Plasminogen activator inhibitor SERPINE1 (PAI1). Polymorphism: 4G/5G (PAI1: 4G/5G; Ins/Del G).
- Methylenetetrahydrofolate reductase MTHFR: C677T (Ala222Val)
- Methylenetetrahydrofolate reductase MTHFR: A1298C (Glu429Ala)
- Methionine synthase MTR: Asp919Gly (A2756G)
- MTRR reductase: Ile22Met (A66G)
Thrombophilia is a predisposition to the formation of thrombi (blood clots) in the lumen of a vessel of a hereditary or acquired nature. Blood clots block blood flow, which causes coronary heart disease, pulmonary embolism, strokes and heart attacks.
Mutations in some genes encoding coagulation factors, platelet factors and fibrinrolysis factors (anti-coagulation system) are responsible for the development of thrombophilia. In healthy people, the coagulation and anticoagulation systems of the blood are in balance, preventing the formation of blood clots and excessive bleeding. In some pathologies, including thrombophilia, the balance is disturbed.
There is no treatment for thrombophilia, but there are effective preventive measures that can prevent the development of complications.
The analysis is a molecular genetic study of the genes for coagulation factors, fibrinolysis, and platelet receptors, changes in the activity of which cause a tendency to increased thrombus formation. Genes of enzymes responsible for folic acid metabolism may be involved in the development of the disease. Mutations in them lead to atherosclerotic and thrombotic changes in blood vessels.
Causes of pathology
As a result of various pathological processes in the body, blood clots can form that block blood flow. Most often, this phenomenon is caused by a mutation in the clotting factor gene (or, as it is also called, Leiden). A mutation in the prothrombin gene can also lead to the development of pathology.
If a patient has even one of these mutations, the risk of thrombophilia increases several times. Various provoking factors can also worsen the situation:
- excess weight;
- taking oral contraceptives;
- smoking;
- sedentary lifestyle;
- oncological pathologies;
- IVF protocols;
- pregnancy period.
References
- Academician RAMS, prof. IN AND. Krasnopolsky, Doctor of Medical Sciences, Prof. V.A. Petrukhin, Ph.D. A.P. Melnikov: Management of pregnant women with thrombophilia. — Russian Bulletin of Obstetrician-Gynecologist 4, 2013
- R.G. Shmakov, P.A. Kiryushchenkov, A.V. Pyregov, M.A. Vinogradova, O.R. Baev, N.E. Kahn, O.G. Pekarev, N.I. Klimenchenko, N.K. Tetruashvili, V.L. Tyutyunnik, Z.S. Khodzhaeva, N.V. Dolgushina, Brief protocol: Study of the hemostatic system during pregnancy and after childbirth, 2015.
- Venous thromboembolism, thrombophilia, antithrombotic therapy, and pregnancy. American College of Chest Physicians evidence-based clinical practice guidelines 8th edition. American College of Chest Physicians – Medical Specialty Society. 2001 January.
- Tsantes AE, et al. Association between the plasminogen activator inhibitor-1 4G/5G polymorphism and venous thrombosis. A meta-analysis. Thromb Haemost 2007 Jun;97(6):907-13.
Antiphospholipid syndrome, genetic thrombophilias in the pathogenesis of the main forms of obstetric pathology
Throughout the existence of scientific obstetrics, the pathology of hemostasis and, as a consequence, its disseminated intravascular coagulation syndrome (DIC syndrome) have always been considered secondary to the main pathological syndromes in obstetrics: preeclampsia, septic shock. It is paradoxical, but despite the fact that most discoveries in hemostasiology are related to obstetrics (DIC syndrome, septic shock as an analogue of the Sanarelli-Schwartzman phenomenon, antiphospholipid syndrome), for a long time they were ignored in obstetrics and were implemented with a great delay compared to other clinical disciplines. The discovery at the end of the twentieth century (1987) of antiphospholipid syndrome (APS) and a number of previously unknown, but most common forms of genetic defects of the hemostatic system, predisposing to various thrombotic complications: factor V Leiden mutation, prothrombin G20210A mutation, methylenetetrahydrofolate reductase mutation (MTHFR C677 T), polymorphism of the plasminogen activator gene (PAI-1 4G/5G), polymorphism of platelet receptors (1993-2000) (Fig. 1) as the main causes of acquired and genetic thrombophilia, allowed us to evaluate the pathogenesis of various complications from a fundamentally new perspective, both in general clinical, and in obstetric and gynecological practice (Fig. 2). Studies on the role of thrombophilia, in particular APS, factor V Leiden mutation, prothrombin G202 10A and MTHFR C677T, which we have conducted since the early 90s, have shown their extremely high frequency not only in patients with various thromboembolic complications, but also typically obstetric complications, such as primordial miscarriages, severe gestosis, premature placental abruption (PNA), intrauterine growth retardation syndrome (IUGR), antenatal fetal death (AFD), etc.
In the last 5 years, we have also intensively begun to study the role of polymorphisms of various thrombophilic genes in the pathogenesis of these complications, which has largely made it possible to judge the relationship between genotype and phenotype, taking into account, of course, specific risk factors for the implementation of latent forms of thrombophilia during pregnancy. Statistical and population-based studies on the role of hereditary hemostatic defects in the etiology of habitual fetal losses and other obstetric complications have been actively conducted since the mid-90s. To date, significant clinical data have been accumulated and an analysis of the results of multicenter studies has been carried out, making it possible to identify hereditary thrombophilias as an independent group of causes of miscarriage. According to generalized data from the world literature, among the causes of recurrent miscarriage, about 7% are chromosomal abnormalities, about 10% are anatomical and about 15% are hormonal (progesterone, estrogens, diabetes or thyroid diseases); approximately 6% of recurrent miscarriages are of unknown origin and about 55-62% are caused by defects in coagulation proteins or platelets. At the same time, it should be noted that about 70% of first miscarriages are caused by chromosomal defects and about 25% of first pregnancies may end in miscarriage. In the world literature of recent years, reproductive losses are increasingly combined into the so-called “fetal loss syndrome”
, including: – one or more spontaneous miscarriages at a period of 10 weeks or more (including non-developing pregnancy); – stillbirth; – neonatal death, as a complication of premature birth, severe gestosis or placental insufficiency; – three or more spontaneous miscarriages at the preembryonic or early embryonic stage, when anatomical, genetic and hormonal causes of miscarriage are excluded. As can be seen from the definition, the structure of fetal loss syndrome, in addition to the previously accepted term habitual miscarriage, also includes non-developing pregnancy, stillbirth, as well as neonatal death as a complication of premature birth, severe gestosis or placental insufficiency. Studying the causes and pathogenesis of the more general fetal loss syndrome compared to habitual miscarriage allows us to take a more global approach to this problem, as well as to develop basic measures to prevent complications of this syndrome in women of reproductive age. If previously recurrent miscarriage (recurrent miscarriage, recurrent spontaneous abortion) was defined as 3 or more pregnancies ending in spontaneous miscarriage, then in recent years, 2 repeated episodes of spontaneous abortion in the early stages are considered sufficient to make a diagnosis of recurrent miscarriage. Of the thrombophilic disorders, only APS was until recently considered as a “thrombophilic” cause of fetal loss syndrome, while the role of genetically determined thrombophilia was not discussed. Although certainly recognized as an independent nosology, in clinical practice APS occurs in combination with other disorders, complicates their course and can become a leading factor determining the outcome of pregnancy. Today, antiphospholipid syndrome (APS) is understood as a symptom complex that combines certain clinical signs and laboratory data: the presence of antiphospholipid antibodies in combination with arterial and venous thrombosis, fetal loss syndrome, immune thrombocytopenia and/or neurological disorders. The syndrome can manifest itself with one or several clinical signs at the same time - up to the development of the so-called catastrophic form of APS, characterized by acute multi-organ failure, reminiscent of that of DIC with the development of acute respiratory distress syndrome, damage to the central nervous system (stroke, stupor, disorientation), myocardial infarction and gastrointestinal organs, adrenal insufficiency, etc. From a modern standpoint, thrombophilia seems to be an integral etiopathogenetic factor in a wide range of complications in general clinical practice, including not only in obstetrics, but also in gynecology, causing complications of hormonal contraception and hormone replacement therapy, infertility and IVF failures , early preembryonic losses (Fig. 2), as well as in oncology, while thrombophilia is a risk factor not only for thrombotic complications and premature death of cancer patients, but also a significant factor in tumor growth and metastasis. In recent years, the non-thrombotic effects of thrombophilia in the pathogenesis of obstetric complications have begun to be considered and studied at the stage of implantation of the fertilized egg and in the early embryonic phase. Implantation, trophoblast invasion and further functioning of the placenta appear to be multi-stage processes of endothelial-hemostasiological interactions with complex autocrine-paracrine regulation, which are objectively disrupted by thrombotic tendencies and in the case of genetic coagulation defects. I would like to note that pregnancy is a condition that can be called a kind of “exam” for the presence of latent acquired (APS) or genetic thrombophilia, since it itself is accompanied by physiological hypercoagulation and contributes to the implementation of latent thrombophilia not only in the form of thrombosis and thrombophilia, but also typically obstetric complications. The presence of additional risk factors may potentiate the effects of thrombophilia in pregnant women; these include cardiovascular diseases, diabetes mellitus, etc. (Fig. 3).
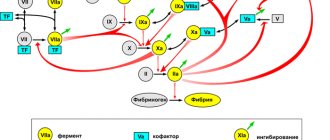
In general, thrombophilia is the result of: a) a decrease in natural antithrombotic defense; b) activation of prothrombotic mechanisms or a combination of these factors. Whatever the initial causes of thrombophilia, the result largely depends on the synergistic effects on the hemostatic system. The most synergistic effects, as can be seen from Figure 4, have APS, the FV Leiden mutation and MTHFR C677T (hyperhomocysteinemia). It should be noted that these defects occur at the same time much more often than AT III and protein C deficiencies (Fig. 4).
Despite the fact that the role of APS, FV Leiden, prothrombin G20210A, MTHFR C677T in the pathogenesis of obstetric complications is currently being widely studied, in fact, isolated publications appear regarding the role of polymorphisms of the PAI-1 4G/4G gene and ACE I/D. We examined not only these two polymorphisms, but also polymorphisms of platelet receptors, fibrinogen, and angiotensin II receptor. Thanks to the wider scope of the study for the presence of thrombophilia, we, on the one hand, obtained a higher percentage of thrombophilia in all examined groups, and on the other hand, we were able to explain the frequent discrepancy between genotype and phenotype. Thus, in confirmation of the above, thrombophilia in general (regardless of genesis) was detected in 71.3% of patients with fetal loss syndrome, up to 80% of patients with gestosis and in 100% of patients with a history of thrombosis and PONRP, which exceeds the frequency of thrombophilia in other similar studies, which assessed only FV Leiden, prothrombin mutation G20210A and MTHFR C677T. According to our study, the role of thrombophilia (including its various forms) is different in the pathogenesis and structure of reproductive losses within the framework of fetal loss syndrome. Thus, the circulation of AFA was more often detected in patients with early miscarriages (43.1%), respectively, in 22.4% of patients with late miscarriages and in 35.7% of patients with early preembryonic losses. At the same time, FV Leiden mutations occurred more often in patients with late miscarriages (15%). The prothrombin G20210A mutation was significantly less common in all groups of reproductive losses (compared to AFA, FV Leiden and MTHFR C677T) and amounted to 4.2% and 3.0% in the groups of early and late miscarriages, respectively. The most common mutation in patients with fetal loss syndrome was the MTHFR C677T mutation, which respectively amounted to 41.2% in patients with fetal loss syndrome. We believe that one of the most important results of the study was the detected high frequency of multigene forms of thrombophilia (two or more defects) in the examined patients with fetal loss syndrome. The total multigene thrombophilia in all patients with fetal loss syndrome was 55.7%: in the group of early miscarriages - 55.6%, in the group of late miscarriages - 62.3%, in the group of early preembryonic losses - 39.3%. At the same time, a characteristic feature of multigene forms of thrombophilia was the high frequency of polymorphism of various thrombophilic genes. When analyzing the clinical manifestations of thrombophilia in patients with fetal loss syndrome, the following feature was also revealed: patients with a history of thrombosis either had a homozygous FV Leiden mutation, or a combination of APA and FV Leiden, a prothrombin G20210A mutation, or the PAI-1 4G/4G polymorphism. In addition, thrombosis was also observed in patients with more than 3 homozygous forms of gene polymorphism (PAI-1, fibrinogen gene and/or platelet receptors). From our point of view, the most striking model for studying the nonthrombotic effects of thrombophilia is early preembryonic losses. We obtained extremely interesting results in the group of patients with preembryonic losses. It is characteristic that the majority of women in this group were initially treated for infertility. Moreover, most of them have already had repeated unsuccessful attempts at in vitro fertilization (IVF). Thrombophilia analysis showed that 60.7% of patients had thrombophilia. In this case, the PAI-1 polymorphism was most often detected (50.0%), including in combination with dysfibrinogenemia and/or ACE “I/D” polymorphism, as well as the MTHFR C677T mutation (35.7%), APA (35. 7%) and multigene forms of thrombophilia (39.3%). It is characteristic that in women with homozygous PAI-1 4G/4G polymorphism, polycystic ovary syndrome and/or metabolic syndrome, there was a significant increase in PAI-1 levels. This important observation suggests that perhaps in the pathogenesis of infertility (or rather, often unrecognized preembryonic losses) in PCOS and metabolic syndrome, in addition to endocrine causes, the presence of simultaneously high levels of PAI-1 plays an extremely important role. During pregnancy, enzymes of the fibrinolytic system occupy a central place in the control and regulation of the physiological process of fibrin deposition, and in some cases, in preventing fibrin deposition in the area of the vascular bed. In general, it should be noted that the physiological course of pregnancy is accompanied by significant changes in the hemostatic system and, in particular, in the area of uteroplacental blood flow. Such physiological adaptation is necessary to ensure at least two important functions: a) integration of the rapidly increasing maternal and fetal blood flows in the area of their “interface” - the placenta; b) the effectiveness of controlling bleeding from the placenta during its separation during childbirth. Changes in the coagulation system during physiological pregnancy are represented by a weak local activation of coagulation in the uterine vascular bed, accompanied by increased synthesis of fibrinogen and other coagulation factors in combination with a slight decrease in the level of natural blood coagulation inhibitors. It is important to note that a decrease in fibrinolytic activity in the uterine bloodstream also affects the state of the coagulation system in the peripheral bloodstream of a pregnant woman. Both intra- and extravascular deposition of fibrin is part of the physiological process during implantation of the fertilized egg and trophoblast invasion in the placental bed. However, recent studies have shown that trophoblast cells are responsible not only for controlling the physiological deposition of fibrin in the placental bed, but also for the increased fibrin deposition that is observed during complicated pregnancy. The process of regulation of fibrinolysis depends primarily on the activity of plasminogen activators (t-PA, u-PA) and on the level of synthesis and secretion of the inhibitor of plasminogen activation and their interaction. The imbalance of uteroplacental fibrinolytic control in IUGR and preeclampsia as a result of increased production of PAI-1 is responsible not only for increased fibrin deposition in the uterine vessels and a decrease in uteroplacental blood flow: it plays an important role in reducing the degree of trophoblast invasion in early pregnancy, which also creates the prerequisites for the further development of gestosis and IUGR. Early preembryonic losses are mainly associated with defects in implantation of the fertilized egg. The implantation process begins on the 6th day after ovulation and is not a one-sided process of active invasion, but a kind of enzymatic “exchange” between the blastocyst and the endometrium, which requires hormonal stimulation and a certain “maturity” of the endometrium. In the process of preparation for implantation, under the influence of progesterone in the endometrium, there is an increase in the content of plasminogen activation inhibitor type 1 (PAI-1), tissue factor (TF) and a decrease in the level of plasminogen activators of tissue and urokinase types, matrix metalloproteases and the vasoconstrictor - endothelin 1. These physiological mechanisms regulation of hemostasis, fibrinolysis, extracellular matrix and vascular tone are aimed at preventing the formation of hemorrhage with further trophoblast invasion. For its part, the blastocyst synthesizes tissue and urokinase-type plasminogen activators and proteases, which are necessary for the destruction of the extracellular matrix during implantation. Their excessive synthesis, in turn, is regulated by human chorionic gonadotropin (HCG). In the process of “dosed” destruction of the matrix under the action of enzymes secreted by the blastocyst, endometrial cells, which contain a certain amount of extravascular fibrin, are not phagocytosed, but are, as it were, “removed” through “contact inhibition”. This phase of the implantation process is called “avascular” or otherwise “histiotrophic”. It should be noted that this is the most vulnerable phase of implantation: often factors such as viruses, toxins, antibodies, etc. can directly affect the usefulness of implantation. From the point of view of the influence of thrombophilia, the most striking example is the PAI-1 polymorphism and other genetically determined defects in fibrinolysis and APS (Fig. 5).
Under conditions of hypofibrinolysis (both as a result of PAI-1 polymorphism and other reasons), desynchronization of the local processes of fibrinolysis and fibrin formation occurs during implantation. In such a situation, the proteases synthesized by the blastocyst become relatively insufficient to destroy the extracellular matrix in the endometrium and penetrate to a sufficient depth. If at the same time there is also circulation of APA, then this aggravates the situation, since: a) APA not only enhance prothrombotic mechanisms and therefore desynchronize the processes of fibrinolysis and fibrin formation, but also b) APA can change the surface preimplantation characteristics of the fetal egg: both charge and and configuration. If we consider this issue in the context of the list of IVF failures, then, from our point of view, in addition to failure to take thrombophilia into account, auxiliary therapy with massive hormone therapy plays an additional negative role. In some cases, patients diagnosed with infertility may experience early preembryonic losses, which are clinically masked by an irregular menstrual cycle. Returning to the effects of thrombophilia, it should be noted that not only the early (avascular) phase of implantation is affected, but also the later stages of implantation (hemotrophic phase) and placentation. The hemotrophic (vascular) phase of implantation begins 12 days after ovulation: lacunae begin to form between the proliferating trophoblast cells, which will subsequently enlarge, merge and transform into the intervillous space of the placenta. It is during this phase that active contact with the maternal plasma begins: the trophoblast penetrates (destroying them) into the thin-walled maternal vessels, from which blood flows freely into the trophoblast lacunae. By 21 days after ovulation, the trophoblast villi are already sufficiently vascularized and it can be stated that uteroplacental blood flow has been established. The factors that ensure trophoblast invasion and normal development of the placenta in the early stages are very diverse: growth factors, cytokines, integrins, adhesion molecules, histocompatibility complex antigens (mainly class 1), etc. Thrombophilia (including those caused by APA) directly or indirectly affects the process implantation and early embryonic stages. Separately, I would like to discuss the role of antiphospholipid antibodies in the pathogenesis of fetal loss syndrome, since the effects of APA are extremely diverse, in contrast to the isolated form of genetic tombophilia, which is apparently due to the greater heterogeneity of various antibodies, united under the general term “antiphospholipid antibodies.” That is why the prognosis is most unfavorable when APS is combined with genetic thrombophilia: in such cases, the prothrombotic tendency becomes disproportionately higher and its implementation is possible not only in the form of thrombotic damage to the microcirculation and fetal loss syndrome, but also in the form of macrothrombosis. Thrombosis of microcirculatory vessels in conditions of thrombophilia is responsible for a wide range of obstetric complications - from IUGR to AGP, stillbirths and PONRP. At the same time, an unfavorable background occurs already from the moment of implantation and differentiation of the trophoblast. The damaging effect of APA can occur in several ways: – the adhesive characteristics of the preimplantation embryo change; – syncytium fusion is disrupted; – the depth of trophoblast invasion decreases; – the production of human chorionic gonadotropin is suppressed; – thrombotic tendencies are enhanced by providing templates for coagulation reactions. The last point explains the positive effect of anticoagulant therapy from the earliest stages. These mechanisms also help explain unsuccessful attempts at artificial insemination and embryo transfer in women with AFA. If the role of thrombophilia in the pathogenesis of fetal loss is currently being intensively studied, the role of thrombophilia in the pathogenesis of preeclampsia has begun to be studied relatively recently. Apparently, this circumstance is due to the fact that gestosis is considered a polyetiological syndrome, and disturbances in hemostasis were considered as secondary phenomena: it is well known that gestosis is accompanied by the development of disseminated intravascular coagulation syndrome. From our point of view, a new era in understanding the etiopathogenesis of gestosis began with the establishment of the role of thrombophilia in the pathology of the processes of implantation of the fertilized egg, placentation and later disorders of uteroplacental perfusion. At present, the leading role of endothelial damage and endothelial cell dysfunction in the pathogenesis of gestosis is no longer in doubt. The main attention of researchers today is focused on the triad of disorders during gestosis: a) violation of trophoblast invasion; b) uteroplacental ischemia; c) generalized activation of endothelial cells or mechanical damage. Although the etiology of gestosis remains an open question, currently insufficient placental invasion, as well as disturbances in trophoblast differentiation and vascular restructuring of the placental bed, are considered important initiating factors. Dysfunction of endothelial cells during gestosis can be the result of various factors: impaired blood flow, hypoxia, lipid peroxidation products - free radicals and atomic oxygen, and other substances. For example, an intravascular increase in the force of the blood shock wave as a result of vasospasm or insufficient restructuring of the spiral arteries disrupts the morphology and function of the endothelium. Hypoxia, as a result of reduced placental perfusion, is a well-known stimulator of the synthesis and secretion of endothelin and endothelial growth factor into the bloodstream. Damage to the endothelium can cause the appearance of highly active derivatives of atomic oxygen, free radicals, as well as antiphospholipid antibodies and cytokines, such as TNF-a, IL-6. It is important to note that in conditions of a chronic pathological process, such as gestosis, such an endothelial response can provoke the development of a vicious circle consisting of vasospasm, microthrombosis, disintegration of the vascular system and pronounced physiological disorders that persist until the initiating factor is removed. The first pathological changes associated with gestosis may appear already in the process of trophoblast invasion, when under normal conditions denudation (detachment) of the endothelial layer of the uterine spiral arteries occurs and its replacement with endovascular cytotrophoblast. Insufficient trophoblast invasion further determines the “endothelial” phenomenon of gestosis, which implies the endothelial genesis of the resulting disorders. We believe that a feature of gestosis as a pathological condition is the development of a vicious circle of changes, which, once they appear, contribute to the emergence of a number of new pathological changes, which, in turn, contribute to the maintenance of the existence and aggravation of old previous changes and the emergence of new disorders. From this point of view, if we accept thrombophilia as a constantly persistent factor in women with genetic thrombophilia or APS, its first effects appear to us as defects in the implantation of the fertilized egg, insufficient depth of trophoblast invasion, defective placentation and, as a consequence, endotheliopathy (Fig. 6) .
All these processes, in turn, cause a further decrease in placental perfusion. A decrease in placental perfusion, in turn, is accompanied by the development of hypoxic changes and even greater damage to the endothelium, which entails an increase in the severity of thrombophilia and vasoconstriction. Thus, the pathogenesis of hypertension during gestosis may consist of at least two components: a) vasoconstriction, as a consequence of a widespread disorder of the vasomotor function of the endothelium; b) a compensatory increase in systemic blood pressure in the mother’s body in response to a decrease in placental perfusion. An additional factor that maintains and aggravates the reduced perfusion of the placenta is thrombosis of the uteroplacental vessels up to a complete block of microcirculation, when both PONRP and AGP are possible, and at earlier stages - FVGR. In general, blockade of microcirculation during gestosis can be truly catastrophic and accompanied by such clinical phenomena as HELLP syndrome, eclampsia, acute renal failure, up to the development of multiple organ failure. All of the above is confirmed by our research. Thus, according to our data, thrombophilia as a complex integral factor in the pathogenesis of the development of gestosis (and, from our point of view, the initial one) was found in 80% of patients with gestosis. Among patients with mild forms of gestosis, thrombophilia was detected in 54%, while in the control group of pregnant women with a physiological course of pregnancy - in 16% (p <0.05). At the same time, the following forms of thrombophilia absolutely prevailed: a) MTHFR C677T mutation, which amounted to 56.8%, while in the group of mild forms of gestosis and in patients with a physiological course of pregnancy – 44 and 12%. It is noteworthy that in the last two groups heterozygous forms predominated, which, apparently, is explained by the lesser severity of the pathological effects of this mutation; b) polymorphism of the PAI-1 gene, which amounted to 49.1%. In the group of pregnant women with mild forms of gestosis, this polymorphism was found in 40%, and in the control group of pregnant women with a physiological course of pregnancy - only 16% (p <0.05). c) multigene forms of thrombophilia, which amounted to 72.5%, while in the group of mild forms of gestosis only 14%, in the group of physiological pregnancy - 4%, and it should be noted that in the two control groups multigene thrombophilia was represented only by polymorphism genes or heterozygous MTHFR C677T, but not the FV Leiden mutation or prothrombin G20210A. Antiphospholipid antibodies were detected in 17.3% in the retrospective group, 16.25% in the prospective group, 10% in the mild gestosis group and 4% in the control group with a physiological course of pregnancy. As for the FV Leiden mutation, it was found in almost 16.4% of patients in the retrospective group and in 15% in the prospective group, in 4% in the group of mild gestosis (and only the heterozygous form) and in 2% in the control group with a physiological course pregnancy (heterozygous form only). From our point of view, the highest percentage of MTHFR mutations also appears to be explained by the endotheliotoxic effect of homocysteine, the level of which increases with the MTHFR C677T mutation (mainly in homozygous forms). In this sense, an interesting analogy: the MTHFR C677T mutation and hyperhomocysteinemia are well-known risk factors for early atherosclerosis, while at the same time, changes in the vascular wall in severe gestosis are characterized by acute atherosis. However, if atherosclerosis forms relatively slowly, then vascular atherosis during gestosis develops catastrophically quickly! Hence the high frequency of so-called emergency conditions - preeclampsia, eclampsia, HELLP syndrome. Since in conditions of hyperhomocysteinemia the main pathogenic role belongs to oxidative stress and free radicals, then, from our point of view, early antioxidant prophylaxis in groups of pregnant women with the MTHFR C677T mutation and hyperhomocysteinemia is very justified, that is, in groups at high risk of developing preeclampsia, until preeclampsia has formed . Another important aspect of the MTHFR C677T mutation in conditions of gestosis is folate deficiency anemia, which further aggravates hypoxia, the course of disseminated intravascular coagulation and thereby contributes to the progression of microcirculatory disorders. When examining both patients in the prospective group and when studying the birth histories of pregnant women in the retrospective group, we found a high incidence of anemia - up to 61.8%. As already indicated, in addition to the MTHFR mutation, a fairly high percentage of PAI-1 4G/4G polymorphism is detected. The pathogenic mechanisms of gestosis are probably associated with more pronounced implantation defects, since fibrinolysis, which plays an important role in the formation of the “mother-placenta-fetus” system, is suppressed. Apparently, this circumstance is also associated with a higher incidence of gestosis in pregnant women with PCOS and a high body mass index. In addition to the fact that they have insulin resistance and an increased level of PAI-1, the presence of PAI-1 gene polymorphism aggravates disturbances in the fibrinolysis system and is, on the one hand, the cause of infertility and early preembryonic and embryonic losses, and on the other hand, preeclampsia . During pregnancy, other additional factors may be present that contribute to an increase in PAI-1 levels (such as hypoxia, cytokines, activation of the renin-angiotensin-aldosterone system, etc.). Separately, the role of angiotensin-converting enzyme in the pathogenesis of gestosis in pregnant women with genetic thrombophilia should be emphasized, since it is well known that this enzyme is one of the key ones in maintaining normal blood pressure levels, and an increase in its level entails an increase in blood pressure. Our study revealed a fairly high frequency of polymorphism of the ACE gene “I/D” (29%), while in the group of pregnant women with a physiological course of pregnancy it was only 12%. Based on the mechanisms of thrombophilia in its various genetic forms, we can make an assumption about more unfavorable combinations of various forms using the example of the PAI-1 4G/4G polymorphism (Fig. 7).
We include as such either a) those forms that potentiate mutual effects through the same mechanisms (that is, act on the same “target”), being individually less thrombogenic, such as, for example, isolated polymorphisms of the PAI-1 genes or ACE; or b) a combination of the most thrombogenic forms of thrombophilia (for example, FV Leiden, prothrombin G20210A, MTHFR C677T, APA), when various mechanisms of thrombophilia are involved, such as: endothelial disorders, increased thrombinemia due to high levels of coagulation factors (as with the Pt G20210A mutation) , decrease in natural antithrombotic resources (with FV Leiden mutation, APS, hypofibrinolysis). Considering thrombophilia as an integral factor of various complications in obstetrics, we considered it correct to combine thrombotic complications and PONRP with the term “acute vascular disorders.” Among those examined with PONRP, the MTHFR C677T mutation, homozygous form (in 70.5%) and hyperhomocysteinemia prevailed, the Leiden mutation was detected in 25.2%, APS - in 37.4% of patients. Due to the key role of thrombophilia in the pathogenesis of fetal loss syndrome, preeclampsia, PONRP and, of course, thromboembolism, the prevention of these complications involves the use of antithrombotic drugs. However, given that disorders associated with thrombophilia and inadequate fibrin formation begin to form at the stage of implantation of the fertilized egg, trophoblast invasion, and placenta formation, it is important to prevent possible later complications even before conception. Our experience shows that pathogenetically based prophylaxis allows in all cases to prevent recurrence of thrombosis and in the vast majority of cases (92-96%) to significantly improve pregnancy outcomes. Moreover, significantly better outcomes occur in patients who receive antithrombotic prophylaxis with Fragmin from early pregnancy and during the fertile cycle. In addition, our experience indicates the high effectiveness of Fragmin in pregnant women with concomitant heart lesions (valve implantation), as well as in the complex treatment of possible shock and shock-like conditions. As for optimizing prevention, in connection with the elucidation of new forms of thrombophilia (such as PAI-1) and the role of fibrinolysis in the early stages of implantation, it seems to us very promising to include hirudotherapy in the process of preparing patients in the fertile cycle.
Treatment of thrombophilia
Complex treatment of thrombophilia is focused on achieving two main goals: prevention of thrombosis and immediate treatment of the provoking pathology. Specialists provide separate treatment for complications, if any are observed.
Thrombophilia therapy includes:
- The use of anticoagulants, thrombolytics and disaggregants;
- Methods of hemocorrection and hydremia;
- Jet blood transfusion (blood transfusion);
- Plasma transfusion;
- Diet therapy;
- Lifestyle correction (especially in the presence of bad habits and physical inactivity);
- Physiotherapy.
Complications are treated and prevented by additional methods. For example, in case of venous insufficiency, it may be necessary to wear a compression device, and in case of intestinal infarction, emergency abdominal surgery may be required to resect the affected areas of the organ.
Interpretation:
- The activity of the factor/enzyme changes as a result of nucleotide substitutions in the gene encoding it: 1. In relation to the coagulation factors of the hemostasis system, a high and medium risk of thrombosis is distinguished: the “neutral” allele is normal activity of the factor, “heterozygote for the mutant allele” is characterized by a change in the activity of the factor and an average risk of thrombosis, “homozygote for the mutant allele” is a significant change in the functional factor activity and a high risk of thrombosis.2. In relation to folate cycle enzymes: “neutral” allele – normal enzyme activity, “heterozygote for a mutant allele” – reduced enzyme activity, “homozygote for a mutant allele” – significantly reduced enzyme activity. Reduced enzyme activity leads to disruption of the metabolic pathway for the conversion of homocysteine, an increase in its content in the blood plasma (hyperhomocysteinemia) and the likelihood of developing pathological conditions.
Sample result (PDF)