All of us and the things around us experience atmospheric pressure created by invisible and almost imperceptible air. It was first successfully measured by the physicist Torricelli in 1634.
An atmosphere weighing about 1 kg acts on 1 cm2. A person does not feel this, since gases, always present in the blood and located in the cavities of the body, balance the external influence.
How to notice atmospheric pressure?
Although gas molecules are odorless and colorless, they constantly interact with the receptors of our skin, compress all objects from all sides, fill voids, and their rapid movement in the horizontal direction, called wind, can knock us off our feet. It is possible to prove that atmospheric pressure exists using simple experiments.
Experiment 1 – “Sippy Cup”
Pour water into the glass to the brim. Cover it with a piece of thick paper and, holding the paper with your palm, quickly turn the glass upside down. Remove your palm. Water will not spill out of the glass, since the atmosphere presses on the paper from below.
Explanation: the phrase “a column of atmospheric air presses on us,” sometimes used, including in school textbooks, is incorrect. It is pronounced by association with the pressure force acting from a solid body. This force acts on bodies located below, and does not act on bodies on the side or, especially, on top of a given body. The pressure of a liquid or gas is another matter.
According to Pascal's law, pressure is transmitted not only to points at the bottom of the vessel, but also to points on the walls and lid. The forces of hydrostatic and atmospheric pressure act perpendicular to the arbitrarily oriented surface of the body in contact with the medium and can have any direction.
The air pressing on the paper from the bottom of a filled glass is proof of the failure of such an association. Interestingly, if the glass is only half filled with water, the remaining air will press with the same force as the outside air, and the paper will not hold the water (and air) in the glass.
Experiment 2 – “Dry out of the water”
Place a coin or metal button on a flat plate and pour in water. The coin will be under water. Our task is to catch the coin with your bare hands without getting them wet.
Light the paper inside a dry glass and, when the air warms up, tip the glass onto a plate next to the coin so that the coin does not end up under the glass. You won't have to wait long. The paper in the glass will immediately go out and the air will begin to cool. As it cools, the water will be drawn into the glass and soon all will gather there, exposing the bottom of the plate.
Explanation: when the air in the glass heated up, it expanded, like all heated bodies, and the excess of its new volume came out of the glass. When the remaining air began to cool, it became insufficient to exert the same pressure in a cold state, to balance the external pressure of the atmosphere. Now the water under the glass experiences less pressure on every centimeter of its surface than in the open part of the plate. It is not surprising that it is driven under the glass, squeezed there by excess pressure from the outside air. The water is pressed in by the air!
On the same topic, look at the experiment of the Galileo program.
Cyclones and anticyclones
Large-scale atmospheric circulation and uneven heating of the earth's surface lead to the appearance of cyclones and anticyclones. Cyclones are large areas with low atmospheric pressure, and anticyclones, on the contrary, with high pressure.
Cyclones are usually more mobile and dynamic than anticyclones. The air in a cyclone rises from the lower part of the atmosphere to higher ones, and since it is warm and humid, dense clouds often form and precipitation occurs.
In anticyclones, the picture is the opposite: the air, on the contrary, descends and is pressed against the earth's surface. This air comes from higher layers of the atmosphere, where the content of water vapor is relatively low. For this and other reasons, the chances of the formation of rain clouds in an anticyclone are low, and the relative and absolute humidity of the air is significantly lower than in a cyclone.
Thus, barometric pressure is one of the main characteristics of the atmosphere. The weather, climate and our well-being depend on it. Barometric pressure is the same as atmospheric pressure. However, now this term is rarely used.
Why don't we feel atmospheric pressure?
Knowing that 1 m3 of air at a temperature of 0° at sea level weighs 1.3 kg, it is easy to calculate that on the roof of a house, having an area of, for example, 100 m², the atmosphere presses with a force of 107 N, which corresponds to the weight of a body weighing 1000 tons. However the roof of the house does not fall in.
The area of the back of a person lying on the beach is obviously greater than 0.2 m2; Consequently, the atmosphere presses on a person’s back with a force greater than 20,000 N, which corresponds to a pebble weighing 2 tons. However, a person does not feel any pressure from above at all.
The “Dry Out of the Water” experiment also shows us evidence of internal pressure balancing the external pressure of the atmosphere.
We do not feel air pressure because atmospheric pressure is evenly distributed on all sides and because inside us there is the same air and liquid pressure, and the body's adaptive abilities constantly balance internal pressure, adjusting it to changes in atmospheric pressure. But adaptations occur only within a short interval.
If people live for a long time at high altitude, then their body adapts to both less oxygen and lower pressure. The highest mountain settlements in the world:
- La Rinconada (Peru) – 5100 m;
- El Alto (Bolivia) – 4150 m;
- Potosi (Bolivia) – 4090 m;
- Lhasa (Tibet) – 3650 m;
- Namche Bazaar (Nepal) – 3450 m;
- in Russia it is Kurush (Dagestan) - 2600 m.
Gold mining village of La Rinconada-Ananey, 5100 m. Author: IJISCAY
But fish living in the depths of the ocean are accustomed to higher pressure, and their body is not able to quickly adapt. Their body has adapted to it, and its internal pressure is much higher than 1 atm. Therefore, when they are taken out of the depths, they explode due to high internal pressure. The same would happen to a person in airless space (in space).
Film on the topic “Atmospheric pressure and human well-being.”
From the history of the discovery of knowledge about weight, air pressure and the invention of the barometer
E. Torricelli , figured out how to measure atmospheric pressure . Together with V. Viviani , a young student of Galileo, he conducted experiments to measure it. Torricelli was also one of Galileo's last students, and based on his guesses, he proved that air has weight and exerts pressure.
Evangelista Torricelli and his barometer. Author: Saperaud~commonswiki
Torricelli was the first to openly oppose Aristotle's dogmas. Speaking about the pump, he stated that
“First of all, the water rises after the piston not at all because “nature is afraid of emptiness,” it’s just that the water is driven into the pump by the pressure that the air exerts on the surface of the river. There is no air in the pump pipe, under the piston, so water enters it until the weight of the water column in the pump pipe balances the external air pressure.”
But he proved it a little later. The experiment he proposed was carried out in 1643. In this experiment, a glass tube about 1 m long was used, sealed at one end. It was filled with mercury and, having closed it with a finger (so that the mercury did not spill out ahead of time), turned it over, and lowered it into a wide cup with mercury.
Some of the mercury poured out of the tube, and a vacuum formed in its upper part (the first real void discovered on Earth - the Torricelli void ). In this case, the height of the mercury column in the tube turned out to be approximately 760 mm (if measured from the level of mercury in the cup). The air pressed on the mercury of the cup and did not allow it to flow out of the tube.
The scientist also guessed that atmospheric pressure is associated with weather changes. Observing the height of the mercury column in the tube, Torricelli noticed that atmospheric pressure was not constant and depended on “warmth or cold.” The column in the tube lowered and then rose, indicating the desired division of the scale. That is why millimeter of mercury (mmHg) is taken as one of the units of pressure. Gravity in Greek is "baros", and Torricelli's device began to be called a barometer .
Operating principle of the Torricelli barometer
Almost simultaneously with Torricelli, another famous scientist of that time, Descartes, . He explained why perfume does not flow out of a bottle with a hole in the bottom when the lid is closed, but does flow out when it is open, precisely because of the difference in air pressure on different surface areas. When the bottle cap is closed, the surface tension of the water at the small opening is able to hold the liquid in the bottle. When the lid is open, it is overcome by the force of air pressure and the perfume begins to flow out. Descartes hypothesized that with height the air becomes thinner, which means its pressure should also decrease.
After Torricelli’s experiments, Descartes instructed the talented French mathematician and physicist Blaise Pascal to test his guess - whether it was true that pressure decreases with height. To do this, he had to climb the mountains with Torricelli's pipe. The descending column of mercury at the height of Mount Puy de Dome confirmed the hypotheses of Torricelli and Descartes.
Pascal concluded:
“the laws of fluid pressure, known since the time of the glorious Archimedes and developed by the Dutchman Simeon Stevin , are in many ways valid for air.”
Air pressure is not noticed by a person, because according to the laws of pressure in liquids and gases, it is directed both to the sides and down.
Meteor dependence what to do?
The movement of mercury by more than one division in 3 hours is a reason for stress in the strong body of a healthy person. Each of us feels such fluctuations in the form of headaches, drowsiness, and fatigue. More than a third of people suffer from weather dependence to varying degrees of severity. In the zone of high sensitivity are populations with diseases of the cardiovascular, nervous and respiratory systems, and elderly people. How to help yourself if a dangerous cyclone is approaching?
Read also: Why do men have blood in their urine?
15 ways to survive a weather cyclone
There's not a lot of new advice here. It is believed that together they alleviate suffering and teach the correct way of life in case of weather vulnerability:
- See your doctor regularly. Consult, discuss, ask for advice in case your health worsens. Always have prescribed medications on hand.
- Buy a barometer. It is more productive to track the weather by the movement of the mercury column, rather than by knee pain. This way you will be able to anticipate the approaching cyclone.
- Keep an eye on the weather forecast. Forewarned is forearmed.
- On the eve of a weather change, get enough sleep and go to bed earlier than usual.
- Adjust your sleep schedule. Provide yourself with a full 8 hours of sleep, getting up and falling asleep at the same time. This has a powerful restorative effect.
- Meal schedule is equally important. Maintain a balanced diet. Potassium, magnesium and calcium are essential minerals. Ban on overeating.
- Take vitamins in a course in spring and autumn.
- Fresh air, walks outside - light and regular exercise strengthens the heart.
- Don't overexert yourself. Putting off household chores is not as dangerous as weakening the body before a cyclone.
- Accumulate favorable emotions. A depressed emotional background fuels the disease, so smile more often.
- Clothes made from synthetic threads and fur are harmful due to static current.
- Keep folk remedies for relieving symptoms in a list in a visible place. It’s hard to remember a recipe for herbal tea or a compress when your temples are aching.
- Office workers in high-rise buildings suffer more often from weather changes. Take time off if possible, or better yet, change jobs.
- A long cyclone means discomfort for several days. Is it possible to go to a quiet region? Forward.
- Prevention at least a day before the cyclone prepares and strengthens the body. Do not give up!
Don't forget to take vitamins to improve your health
Atmospheric pressure is a phenomenon that is absolutely independent of humans. Moreover, our body obeys it. What the optimal pressure should be for a person is determined by the region of residence. People with chronic diseases are especially susceptible to weather dependence.
How is atmospheric pressure measured?
The Torricelli barometer is still used today. This simple device helps determine the approximate altitude above sea level. Climbers take it with them high into the mountains. A barometer is a mandatory instrument in the cockpit of every aircraft, be it an airplane or an Earth satellite. Nowadays, his “brothers” go down to the bottom of the seas. From altimeters they turned into depth gauges.
Over more than three centuries, barometers have changed: they have become automatic, self-recording, and have learned to control other mechanisms.
Mercury barometer measures atmospheric pressure with the greatest accuracy
Old mercury barometers.
Author: GianniG46 At meteorological stations, atmospheric air pressure is measured by the same mercury barometers , since they have the greatest accuracy. They work on the same principle as Torricelli's invention.
When measuring pressure, corrections are made for temperature, since as temperatures rise, mercury and the barometer scale expand. In practice, they use a ready-made correction table, which immediately gives the desired value.
Membrane barometers
Diaphragm pressure gauges are also used to measure atmospheric pressure . The simplest membrane pressure gauge is shown schematically in Fig. 1.
Rice. 1. Membrane barometer
A thin elastic plate-membrane 1 hermetically closes the box 2 , from which part of the air is pumped out. 3 is connected to the membrane , turning around O at an angle depending on the degree of deflection of the membrane, which in turn depends on the difference in the measured air pressure force outside the box and inside the box.
Such pressure gauges are called aneroid barometers . They are calibrated and verified using a mercury barometer. They are less accurate, but more convenient to use because they do not contain mercury. When determining pressure with an aneroid, three corrections are made (to the scale, to the temperature, and additionally to the device), specified in the device certificate. An aneroid can only give reliable readings if it is subjected to careful testing from time to time.
Aneroid barometer. Image by Wolfgang Eckert from Pixabay
The aneroid can be calibrated directly to the height of the atmosphere. Such aneroids are called altimeters ; or altimeters , they are used in airliners and allow the pilot to control the flight altitude.
Bulova B-11 altimeter, from a fighter aircraft. Author: Dosimeter
To continuously record changes in atmospheric pressure, a recording device is used - a barograph . The receiving part of the barograph is several small aneroid boxes connected to each other.
Other devices
Hypsothermometer
(
hypsometer
,
thermobarometer
,
barothermometer
) - a device for measuring atmospheric pressure by the temperature of a boiling liquid (usually water). It is more accurate than the aneroid.
Consists of a boiler and a thermometer with a scale divided into 0°.01. This device is usually used in expeditionary conditions for barometric leveling.
Stormglass is a chemical or crystalline barometer consisting of a glass flask or ampoule filled with an alcohol solution in which camphor, ammonia and potassium nitrate are dissolved in certain proportions.
This chemical barometer was actively used during his sea voyages by the English hydrograph and meteorologist, Vice Admiral Robert Fitzroy , who carefully described the behavior of the barometer; this description is still used today. Therefore, stormglass is also called the “Fitzroy Barometer”. In 1831–1836 _ Fitzroy led the oceanographic expedition on HMS Beagle, which included Charles Darwin .
In spring and autumn, a sharp drop in barometer readings portends windy weather. In summer, in extreme heat, it warns of a thunderstorm. In winter, especially after prolonged frosts, a rapid drop in the mercury column indicates an upcoming change in wind direction, accompanied by thaw and rain. On the contrary, an increase in the mercury column during prolonged frosts foreshadows snowfall.
Ecology DIRECTORY
BAROMETRIC PRESSURE. An obsolete synonym for atmospheric pressure.[...]
Barometric pressure is made up of the sum of the partial pressures of dry air and water vapor contained in the air. These partial pressures are called partial. [...]
Barometric pressure does not appear to be a direct limiting factor, although some animals undoubtedly respond to changes in it; however, barometric pressure is directly related to weather and climate, which have a direct limiting effect on organisms. In the ocean, hydrostatic pressure plays a large role, since its gradient from the surface to the bottom is enormous. With immersion in water, for every 10 m, the pressure increases by 1 atm.[...]
Water pressure is usually measured using a Bourdon pressure gauge (Fig. 4.2, c). A hollow metal tube of elliptical cross-section is bent along a circular arc, and an arrow is attached to its end using an appropriate device. As the pressure inside the tube increases, the elliptical cross-section tends to become circular and the free end of the tube moves upward. The pressure gauge scale can be graduated in kPa. Pressure gauges measure pressure relative to atmospheric pressure. Atmospheric, or barometric, pressure is caused by the attraction of a large mass of air above it to the Earth. Under standard atmospheric conditions, barometric pressure at sea level is 101.3 kPa. If the measured pressure is greater than atmospheric pressure, the resulting relative value is sometimes called gauge pressure. If the measured pressure is less than atmospheric pressure, it is called vacuum. Absolute pressure is a term used to denote pressure that includes atmospheric pressure, that is, it is pressure relative to zero level. [...]
Synonym: air pressure. Obsolete synonym: barometric pressure.[...]
Barometric pressure is determined, the value of which, in the absence of a laboratory barometer, can be obtained at the nearest meteorological station. At the beginning of the test, the temperature of the product being tested is noted.[...]
Correction for barometric pressure should be made in ■disputable cases or if the barometric pressure value deviates by more than 13 mm from the normal pressure of 760 mmHg. Art.; in this case, corrections must be made to the flash point in accordance with table. 2.[...]
In technology, the technical atmosphere is accepted as a unit of barometric pressure, equal to a pressure of 1 kg per 1 cm2 or the weight of a column of mercury 736 mm high or water 10,000 mm high. [...]
Similar phenomena occur when barometric pressure changes.[...]
Until now, we have considered the content of gases in water at a barometric pressure of 760 mm, but with a change in pressure, the content of gases also changes, namely, with a decrease in pressure, gases are released by water, and, conversely, with an increase, water absorbs gases. Therefore, the gas content can change in over a short period and is especially noticeable before thunderstorms, to which fish (eels) react, and the loach even serves as an indicator of approaching thunderstorms; on the other hand, it becomes clear why reservoirs located at heights contain less gas than those located in lowlands. But the influence of pressure is far from being as significant as the influence of temperature. Nevertheless, this influence is strongly detected at depths, which is understandable, taking into account that every ten meters of depth in fresh water increases the pressure by 0.97 atmospheres, and therefore the gas content can be determined by multiplying the gas volume of the upper layer of water by the number of decameters of depth. Thus, at a depth of 1000 meters or more, the release of gas could be enormous; but in reality this does not happen for the reason that while the upper layer of water is saturated with air directly from the atmosphere, the lower layers of water receive it from the upper layers by diffusion. But since this can only happen when the upper layers are oversaturated, and when there is a minus against the norm, reverse diffusion occurs, then the lower layers cannot have more air than the upper layers due to diffusion. In addition, the supply of air to the lower layers occurs due to the vertical circulation of water, caused by a decrease in the temperature of the surface layer, and since the water falling due to its gravity is of a lower temperature, the lower layers in this case should be richer in air, and with continued cooling of the upper layers for in deep autumn and winter, on the contrary, the upper layers are richer.[...]
U2o=K, 293RD273+[...]
The table shows the values of absolute humidity q, g/m3, at a barometric pressure of 101,325 Pa, or 760 mm Hg. Art., and dew point t, °C. [...]
Since air samples for analysis are taken at different temperatures and barometric pressure, it is necessary to bring the sampled volume of air to normal conditions (0 ° C and 760 mm Hg). [...]
Since air samples for analysis are taken at different temperatures and barometric pressure, it is necessary to bring the sampled volume of air to normal conditions (0 ° C and 760 mm Hg). [...]
Turn on the magnetic stirrer and record the start time of the experiment. At the same time, barometric pressure is noted. As oxygen is consumed by the mixture of waste liquid and activated sludge, a negative pressure is created in the respiration flask, recorded by pressure gauge 2. When the difference in the levels of the manometric liquid reaches 20-30 mm, oxygen is added from an oxygen burette. To do this, note the reading of the oxygen burette, open valve 18 and, by adjusting the position of the cup with mercury 22, add oxygen to the respiration flask until the difference in the levels of the pressure gauge is 2 to 0. Oxygen is added with the magnetic stirrer running. After this, close tap 18 and record a new burette reading. By the difference in oxygen burette readings, the amount of added oxygen is judged.[...]
However, altitudes above 3300 m above sea level are a different matter. At these altitudes, there is low barometric pressure and, as a consequence, low partial pressure of inhaled and exhaled gases, a large difference in day and night temperatures, increased solar radiation and the density of high-energy heavy particles in the atmosphere. Significant changes occur in the human body: the partial pressure of gases, especially oxygen, drops in the arterial blood, and the oxygen transport function of the blood changes. [...]
Vacuum suction lift Hb is the maximum difference between the barometric pressure and the pressure in the suction pipe.[...]
DECOMPRESSION DISORDERS are pathological phenomena that occur with rapid changes in barometric pressure and are characterized by the magnitude of the difference, time, speed and its frequency. See also decompression, explosive decompression, barocaveopathy.[...]
PRESS CHAMBER - a hermetically sealed airtight chamber for artificially changing barometric pressure.[...]
Significant changes in the animal body can occur under the influence of an increase or decrease in barometric pressure. It is known that as the terrain rises above sea level and the barometric pressure drops (as a result of air rarefaction), along with rapid breathing and heartbeat in the blood of animals, the number of red blood cells and the hemoglobin content increase. Thus, according to I.A. Polyakov and E.F. Borshchevskaya, fat-tailed sheep from the high mountain regions of the Alma-Ata region, compared with the same sheep bred in deserts, were distinguished by more developed hearts (by 18%), lungs (by 25 %), kidneys, liver and less developed skin. Adaptive changes in animals living in high mountain areas are also expressed in an increase in those indicators of red blood, which are associated with the intake of the required amount of oxygen into the body (see Table 91). However, with a significant decrease in atmospheric pressure and a sharp lack of oxygen, adaptive changes in blood circulation and respiration become ineffective; Due to the accumulation of incomplete combustion products in the body, animals develop mountain sickness, which sometimes takes severe forms with a fatal outcome. [...]
A person lives in a changing environment: fluctuations in temperature, humidity, barometric pressure, etc. However, the internal environment of a person, and primarily body temperature, remains constant. Schematically, our reaction to external influences can be represented as a sequential response in three stages. The first step is genes, carriers of the physiological and mental characteristics we inherited from our parents. Chemical messengers - hormones - are the second stage, they control the cells. But the third stage, the brain, intervenes in these actions. It processes information coming from the environment and corrects the influence of the hormonal system.[...]
In addition, air density varies inversely with absolute temperature and directly with barometric pressure. Thus, for the same volume, resistance and power are proportional to the density of the air.[...]
Gas exchange between the soil and the atmosphere is caused by the diffusion of gases, changes in soil temperature during the day, changes in barometric pressure, displacement of air from the soil during precipitation or irrigation, and also by wind. The meaning of these phenomena is ambiguous. Some researchers consider the main constant factor in gas exchange to be the diffusion of gases (Rommel), others - the change in soil temperature during the day - the “breathing” of the soil (A. G. Doyarenko). However, in the soil, air exchange also occurs simultaneously with diffusion.[...]
The temperature difference was defined as the difference between the temperature of the continuous medium and the boiling point of the agent at a given barometric pressure. As follows from the calculated and experimental data, the influence of the hydrostatic column of liquid, which changes from 0.3 to 0.0 m during the floating of the bubble, on the boiling point of the agent, and therefore on the temperature pressure at AT > 3° C, can be neglected. When AT is close to zero, this influence is significant and can lead to a clearly erroneous result - an increase in the heat transfer coefficient with a decrease in AT. [...]
ISOLINES [otgr. isos - equal] - lines of equal values of k.-l. values on a geographical map or diagram: wind speeds - isoanemones; barometric pressure - isobars; underwater depth contours - isobaths; salinity - isohaline; precipitation amounts - isohyets; temperatures - isotherms, etc. WASTE CONTAINMENT - storing waste in such a way as to effectively prevent its leakage into the environment or allow this leakage to an acceptable extent. [...]
Deviations of the actual oxygen concentration from the equilibrium are caused by: a) physical influences, for example, a sharp change in barometric pressure, a change in water temperature, aeration of water in dams; b) physicochemical and chemical influences, for example, the absorption of oxygen during electrocorrosion of metals and its consumption for the chemical oxidation of substances contained in water or in contact with it; c) biochemical influences that predominate in natural conditions, such as the consumption of oxygen during aerobic microbial decomposition of organic substances or, conversely, the release of oxygen during the absorption of CO2 by organisms. [...]
The main reason for the penetration of air through the enclosing structures is convective gas exchange, which occurs due to the difference in pressure inside and outside the chamber. This is due to the switching on and off of refrigeration equipment, during which the products stored in the chamber are either cooled or heated, as well as changes in the barometric pressure of the external atmosphere, the amount of solar radiation, etc. Parallel to the convective exchange, diffusion gas exchange occurs in the chambers, caused by the difference in the partial pressures of individual gases inside and outside the chamber. The intensity of convective gas exchange is 5-10 times higher than diffusion. The lower the degree of tightness, the more difficult it is to create a gas environment of the desired composition, especially by biological means. It is necessary to take into account the following: if the chamber is depressurized after 3-4 months of storage of fruits and vegetables, then in the future, even with proper gas insulation of the room, it is impossible to create the required composition of the gas environment in it biologically, since by this time the fruits and vegetables have minimal intensity breathing.[...]
For the normal functioning of a biological system, very specific environmental parameters are needed: humidity, temperature, barometric pressure (this has been known for a long time), as well as the characteristics of the magnetic field (this has become clear recently). Deviations of environmental parameters, in particular magnetic induction (or intensity), from the average values to which a given biological species has adapted in the process of evolution leads to disruption of biological processes.[...]
Increased natural ventilation in the presence of connections between the sewer network and the atmosphere is caused by wind, wastewater movement, changes in barometric pressure and pipe filling. [...]
If a person is at an altitude above 5000 m above sea level, he feels unhealthy, and cannot live permanently at such an altitude. Thus, an altitude of more than 5500 m and a barometric pressure of 500-370 mm Hg. Art. are limiting factors.[...]
For the experiments, loose cotton filters with a thickness of 24 cm were used, which completely guaranteed the absorption of all light, heavy and super-heavy airborne particles and at the same time did not noticeably reduce the barometric pressure in the experimental chamber at a given air speed. [...]
An important new advantage of the multi-purpose instrument design is the development of an optical correction servo system, which eliminated the need for careful control of temperature and barometric pressure in the laboratory. Such a servo system is based on continuous monitoring of the position of the mercury line using a photomultiplier, a servo amplifier and a servo motor that controls the deflection plate at the entrance slit [50]. Except for a few moments of exposure, the servo system continuously controls the plate and corrects changes in the position of the exit slit as small as one hundredth of its width. The electronic data reading unit in such a device controls a standard teletype with a printing machine and is tightly connected to the computer. There is a special block for logarithmic data reading; it is ideal for ES and also provides range adjustment and single-point standardization for maximum accuracy.[...]
The processes of exchange of soil air with atmospheric air are called aeration or gas exchange. Gas exchange occurs through a system of air-bearing soil pores that communicate with each other and with the atmosphere. Gas exchange is caused by several factors: diffusion, changes in soil temperature and barometric pressure, changes in the amount of moisture in the soil under the pressure of precipitation, irrigation, evaporation, the influence of wind, changes in the level of groundwater or high water. [...]
Initial data. The diameter of the apparatus is 1.4 m. The composition of the liquid in the apparatus,% (may): water - 40, benzene - 30 and dichloroethane - 30. The external environment is air mixed with ammonia. Air humidity f = 50%. Ammonia concentration in air CNHj = 10 mg/m3. Liquid temperature t = 40 °C. No heat is supplied to the device. Barometric pressure of the external environment B = = 101,325 Pa. Outdoor temperature t = 16 °C. [...]
According to GOST 12.1.014-84, the relative error in measuring the concentration of harmful substances in the air with indicator tubes should not exceed ±35% in the range from 0.5 to 2.0 MPC and ±25% at concentrations above 2 MPC under climatic conditions: ambient temperature environments from 15 to 30 °C; relative humidity from 30 to 80%; barometric pressure from 90 to 104 kPa (675-780 mm Hg). An increase in error up to ±60% is allowed in the range from 0.5 to 1.0 MPC, which must be indicated in the relevant regulatory and technical documentation for indicator tubes. This is due to the fact that in the USSR, for a number of substances, the maximum permissible concentrations of harmful substances in the air of the working area are lower than in foreign countries, and higher metrological characteristics of indicator tubes for monitoring 0.5 MPC of a wide range of harmful substances cannot always be achieved .[…]
To check the “0” scale, the thermometer is immersed for 15 minutes in a funnel filled with crushed ice. To check the 100° division of the scale, thermometers are placed in vapors of boiling distilled water so that there is 2 ohms from its surface to the reservoir. In this case, it is necessary to make corrections, since the boiling point of water increases with increasing barometric pressure. The correction is made according to the Regnault formula: 100-0.037 (760 - B), where 0.037 is the empirical coefficient, B is the barometric pressure at the time of testing. [...]
With the development of the study of electricity in general and atmospheric electricity in particular, with the establishment of research methods and the invention of methods for measuring the main factors of atmospheric electricity, no one had any doubt that the atmosphere influences living organisms not only by its temperature, humidity, barometric pressure and other meteorological or aerodynamic factors, but also its electricity - electric field and electric charges.[...]
Air 36,000 kg/h with initial parameters = 30° C; ¡х = = 15.75 kcal/kg; (pt = 50% is processed in a typical KT30 type irrigation chamber with a cross section of 3.34 m3. Barometric pressure 730 mm Hg. Determine the final parameters of air and water, the amount of total and sensible heat. [...]
Technical data of the EG-152-003 analyzer are as follows. Measuring range 0—10 mg/l Og. The main error of readings is 6% of the measuring range. This error is ensured under the following conditions: temperature of the test water 20 ± 0.2 ° C, salt content no more than 0.1 g/l; pH = 7 t-8; water flow speed at the location of the sensor installation is at least 0.6 m/sec; air temperature 20 ±2° C; relative humidity 55 ±25%; barometric pressure 760 ± 25 mm Hg. Art.; supply voltage 220 ± 2% V; power consumption 150 s.[ …]
The regulations governing the activities of most industrial firms in France contain clauses strictly prohibiting the release into the atmosphere of thick smoke, fumes, dust, toxic or corrosive gases that could harm public health and safety, agriculture, public monuments and works of art. There is a law from April 1932 that aims to reduce emissions of harmful industrial gases. These rules do not formulate with sufficient precision the question of the degree of air pollution that is harmful. Only in the Seine department there are rules governing the combustion of coal and oil in industrial furnaces. According to these rules, during normal operation of furnaces, the density of smoke should not exceed No. 1 on the Ringelmann scale, and when drilling boilers, the release of smoke of greater density is permitted for a period not exceeding 5% of the total operating time of the furnace. Flue gases should not contain more than 1% (by volume) carbon monoxide and more than 2% 502. The dust concentration in the flue gases should not exceed 1.5 g/m3 at a temperature of 0° and a barometric pressure of 760 mm Hg. Art., and the absolute emission is more than 300 kg/hour. Industrial pipes must be of sufficient height to provide a satisfactory degree of dilution and dispersion of gases and dust in the atmosphere. Based on these rules, satisfactory results were not achieved, which prompted the government to study the problem of air pollution.[...]
Patterns in changes in atmospheric pressure and how to use this knowledge
Almost the entire mass of the Earth's atmosphere is concentrated in a layer up to approximately 50 km high. Upon reaching an altitude of 50 km, the acceleration due to gravity decreases by only 1.5% compared to the acceleration at sea level; therefore, we can assume that within the entire 50-kilometer layer of the atmosphere, the acceleration of gravity remains equal to g = 9.8 m/s2.
Representing atmospheric air as a continuous medium, we, of course, must not forget that in reality it is a gas. Pressure is a statistical quantity expressed through the square of the speed of their chaotic movement averaged over many molecules. The force of pressure on any real or mentally selected area in a gas is due to the chaotic bombardment of this area by many molecules.
Pressure decreases with altitude and increases when descending into deep mines. The reason is the rarefaction of air (decrease in density) with ascent and compaction with descent, because it is attracted by the earth and the bulk of its mass is concentrated around it. In the lower troposphere, pressure decreases with altitude by approximately 1 mm for every 10.5 m. This makes it possible to determine the height of a place using a barometer-altimeter.
How does atmospheric pressure change with altitude?
In fact, this pattern is observed only up to a height of 1 km. The distance in meters that one must rise or fall in order for the atmospheric pressure to change by 1 mb is called pressure level . The pressure level at an altitude from 0 to 1 km is 10.5 m, from 1 to 2 km – 11.9 m, at an altitude of 2-3 km the pressure level is 13.5 km. The pressure level depends on the temperature. It is greater in warm air. The barometric formula is described more precisely here: https://ru.wikipedia.org/wiki/
In practice, special tables are often used that allow more or less approximately obtaining data on heights. But to solve problems that do not require high accuracy, you can also use the average value. You can estimate the pressure from the difference in height, and calculate the height from the difference in pressure.
Problem 1
Climbers climb a mountain whose height is 5100 m. At the foot of the mountain, the pressure is 720 mm Hg. Art. What pressure will there be at the top?
Solution:
When ascending 10.5 m, the pressure decreases by 1 mmHg. Art.
1) Find out how many mm. rt. Art. The pressure will decrease when climbing this mountain. 5100:10.5=486 (at 486 mmHg)
2) Find out what the pressure will be at the top. 720-486=234 (mm Hg)
Answer: At the top there will be a pressure of 234 mmHg. Art.
Problem 2
Determine at what altitude the plane flies if the pressure outside is 450 mm Hg. Art., and at the surface of the Earth 750 mm Hg. Art.
1) Determine the difference in pressure. 750-450=300 mm Hg. Art. - the plane rose 10.5 meters so many times.
2) Find out how many meters the plane rose. 10.5 X 300 = 3150 (m)
Answer: the plane is at an altitude of 3150 m.
Problem 3
At the foot of the hill, the barometer shows pressure - 761 mm Hg. Art., and at the top - 761 mm Hg. Art. What is the height of the hill?
The problem is solved according to the same principle as the previous one.
1) 761-750=11 (mm Hg)
2) 11 X 10.5 = 115.5 (m)
Answer: the height of the hill is 115.5 m.
The influence of pressure and altitude on the gas composition of air
The effect of barometric pressure is not limited to its effect on human well-being. With a decrease in atmospheric pressure at altitudes, the gas composition of the atmosphere also changes. First of all, the percentage of water vapor decreases, which is associated with its freezing and condensation. Any gases that are heavier than air (for example, carbon dioxide) decrease faster as you gain altitude than lighter ones (for example, methane). This may affect the nature of the greenhouse effect of the atmosphere at different altitudes, which rapidly decreases with altitude due to a decrease in the content of water vapor. Since methane penetrates to a greater extent into the higher layers of the atmosphere, the nature of the greenhouse effect it causes and the impact on the climate will not be exactly the same as that of carbon dioxide.
Atmospheric pressure is constantly changing
Air density depends on temperature, and temperature is the main reason for changes in air pressure. The pressure of warm air is less than that of cold air. This is explained by the fact that when heated, air, like all objects, expands, its volume increases and it flows into the upper layers in place of less heated air, which leads to a decrease in pressure near the earth's surface.
On climatic and synoptic maps, points with the same pressure indicators, normalized to sea level, are connected by isolines called isobars . Isobars can be closed or open. A system of closed isobars with low pressure in the center (H) is called a pressure minimum , or a cyclone . A system of closed isobars with increased pressure in the center (B) is called a pressure maximum , or anticyclone . Unclosed systems of isobars - baric ridge, trough and saddle .
All pressure areas are divided into two groups: permanent and seasonal (retain characteristic pressure features during a certain period of the year).
Pressure belts on Earth
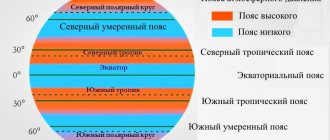
Pressure on Earth is distributed zonally. In a generalized form, this zoning is represented in the form of belts:
- at the equator there is a low pressure belt - an equatorial depression;
- to the south and north of the equator to 30-40° latitude - a belt of high pressure;
- at 60-70° N. and Yu. w. – low pressure belts;
- Subpolar regions – low pressure.
Atmospheric pressure belts on Earth
In fact, the real picture of pressure distribution on the earth's surface is much more complex.
Constant pressure regions
The equatorial belt of low pressure remains constant, only shifting its axis following the Sun. In July it moves to the Northern Hemisphere at 15-20° N. sh., in December - to Yuzhnoye, at 5° south. w. In winter, a continuous belt of high pressure appears over the ocean and over land. In summer, high pressure remains over the oceans, and a thermal depression and low pressure form over land. The pressure maxima of Antarctica and Greenland are also constant.
Above the ice-free oceans and warm currents of the temperate zone, both in winter and summer, pressure minima are clearly expressed:
- Icelandic;
- Aleutian.
Seasonal pressure areas
30-40° latitude
Only in winter is there really a belt of high pressure here. In summer, it becomes low over the mainland, but over the oceans, which warm up slowly, the pressure remains high and even increases. In other words, baric maxima persist here throughout the year only over the oceans:
- North Atlantic;
- North Pacific;
- South Atlantic;
- South Pacific;
- South Indian.
Temperate and subpolar
In the temperate and subpolar latitudes of the northern hemisphere, where oceans and continents alternate, the pressure over land and water is different, especially in winter. Over land there is a minimum in summer, and a maximum in winter. In summer, pressure is low throughout the entire zone. In winter, pressure is high over cooled continents, and seasonal pressure maxima occur here:
- Asian, centered over Mongolia;
- North American (Canadian).
Daily fluctuations in atmospheric pressure
There is also a daily fluctuation in pressure. There is one maximum at night, and one minimum during the day. Twice a day, in the morning and in the evening, it increases and decreases the same number of times, after midnight and after noon.
The change in pressure during the day is associated with air temperature and depends on its changes. Annual changes depend on the heating of continents and oceans in the summer and their cooling in winter. In summer, an area of low pressure is created on land, and an area of high pressure is created over the ocean.
The minimum value of atmospheric pressure - 641.3 mm Hg or 854 mb - was recorded over the Pacific Ocean in Hurricane Nancy, and the maximum - 815.85 mm Hg. or 1087 MB – in Turukhansk in winter. The maximum pressure in Russia was recorded in the Krasnoyarsk Territory in 1968 – 870 mm Hg. Art.
All pressure systems have a great influence on air currents, weather and climate over large areas. We'll talk about the winds they cause next time.
How does pressure affect a person?
The human body consists largely of liquids, as well as gases and their mixtures. This combination successfully resists air pressure. Human blood vessels create their own pressure, the strength of which is influenced by external forces. It was calculated that the ideal indicators of atmospheric pressure are a set of indicators:
- 760 mmHg Art.;
- t = 0˚C;
- the location of the person should not be above sea level.
But not everyone can achieve simultaneous fulfillment of all conditions. Settlements are located, as a rule, above sea level. In addition, each territory has its own relief and its own temperature regime. For example, in high-mountain resorts low pressure prevails, because the layers of the atmosphere high in the mountains become thinner.
However, the mark of 760 mm Hg. Art. is the standard. If barometer readings exceed this value, they speak of high pressure, and in the opposite situation, low pressure.
The decrease in pressure is accompanied by a decrease in the level of oxygen in the air and an increase in the concentration of carbon dioxide. This affects the condition: the tissues of the cerebral cortex do not receive enough oxygen, shortness of breath and insurmountable weakness occur. The heart, in order to compensate for the lack of oxygen, begins to pump blood more intensely, and along with the increase in the speed of the blood volume being pumped, blood pressure increases.
High barometric pressure can cause headaches, general malaise and disability.
Test to consolidate learned material
Test on the topic: “Atmospheric pressure”
Sources:
- Tomilin A. N., Terebinskaya N. V. Why nothing? Essays. /L., “Det. lit.”, 1975.
- Ya. I. Perelman. Entertaining tasks and experiments. - M.: “Children's Literature”, 1972.
- Physical Geography: Reference. aid for preparation. dept. universities/G. V. Volodina, I. V. Dushina, S. G. Lyubushkina and others; Ed. K.V. Pashkanga - M.: Higher. school, 1991.
- Tarasov L.V. Atmosphere of our planet. - M.: FIZMATLIT, 2012.
- Savtsov T.M. General geography: Textbook. aid for students higher ped. textbook institutions - M.: Publishing House, 2003
- Dronov V.P. Geography. 5-6 grades: Textbook/V. P. Dronov, L. E. Savelyeva. 5th ed., stereotype. - M.: Bustard, 2015.
- Geography grades 5-6: textbook. for general education institutions / A. I. Alekseev, E. K. Lipkina, V. V. Nikolina, etc.; Edited by A. I. Alekseev. - M.: Education, 2012.