Patent ductus arteriosus
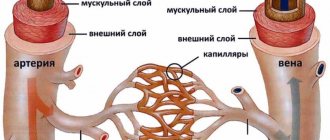
The patent ductus arteriosus is located in the upper floor of the anterior mediastinum; it originates from the aortic arch at the level of the left subclavian artery and flows into the pulmonary trunk at its bifurcation and partially into the left pulmonary artery; sometimes there is a right-sided or bilateral ductus arteriosus. The ductus botallus can have a cylindrical, cone-shaped, fenestrated, aneurysmal shape; its length is 3-25 mm, width – 3-15 mm.
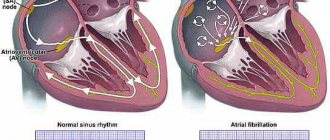
The ductus arteriosus and the patent foramen ovale are necessary physiological components of the fetal circulation. In the fetus, blood from the right ventricle enters the pulmonary artery, and from there (since the lungs do not function) through the ductus arteriosus into the descending aorta. Immediately after birth, with the newborn's first spontaneous breath, pulmonary resistance falls and pressure in the aorta rises, leading to the development of shunting of blood from the aorta into the pulmonary artery. The inclusion of pulmonary respiration promotes spasm of the duct due to contraction of its smooth muscle wall. Functional closure of the ductus arteriosus in full-term infants occurs within 15-20 hours after birth. However, complete anatomical obliteration of the Botallian duct occurs by 2-8 weeks of extrauterine life.
A patent ductus arteriosus is said to exist if its functioning does not stop 2 weeks after birth. Patent ductus arteriosus is a pale type defect because it causes oxygenated blood to be discharged from the aorta into the pulmonary artery. Arteriovenous shunting causes additional volumes of blood to enter the lungs, congestion of the pulmonary vascular bed and the development of pulmonary hypertension. The increased volume load on the left parts of the heart leads to their hypertrophy and dilatation.
Hemodynamic disturbances with a patent ductus arteriosus depend on the size of the message, the angle of its origin from the aorta, and the pressure difference between the systemic and pulmonary circulation. Thus, a long, thin, tortuous duct, extending at an acute angle from the aorta, resists the reverse flow of blood and prevents the development of significant hemodynamic disorders. Over time, such a duct can become obliterated on its own. The presence of a short, wide open ductus arteriosus, on the contrary, causes significant arteriovenous discharge and pronounced hemodynamic disorders. Such ducts are not capable of obliteration.
Publications in the media
Patent ductus arteriosus (PDA) is a vessel through which pathological communication between the aorta and the pulmonary artery (PA) remains after birth. In healthy children, the functioning of the duct stops immediately after birth or continues in a sharply reduced volume for no more than 20 hours. Subsequently, the ductus arteriosus gradually obliterates and turns into an arterial ligament. Normally, obliteration of the duct ends in 2–8 weeks. The ductus arteriosus is considered an anomaly if it is functioning 2 weeks after birth.
Statistical data : PDA is one of the most common defects (6.1% of all congenital heart defects in infants, 11–20% of all congenital heart defects diagnosed in the clinic, 9.8% according to autopsies); The ratio of male to female is 1:2. Etiology: familial cases of the defect have been described; often the mother has a history of rubella, herpes, influenza at 4–8 weeks of pregnancy; Prematurity and neonatal respiratory distress syndrome, hypoxia of the newborn with a high Pg content are predisposing factors. Pathophysiology. The direction of blood discharge is determined by the pressure difference between the aorta and the pulmonary artery and depends on the resistance value of the pulmonary and systemic vascular beds (while the pulmonary vascular resistance is lower than the systemic resistance, blood is discharged from left to right; when pulmonary resistance predominates, the direction of shunting changes). With large PDA sizes, changes in the pulmonary vessels occur early (Eisenmenger syndrome).
Clinical picture and diagnosis Complaints: fatigue, shortness of breath, a feeling of interruptions in the heart, frequent infections, paradoxical embolism. Objective examination • Delay in physical development • Pallor of the skin, unstable cyanosis when screaming, straining • Symptoms of “drumsticks” and “watch glasses” • Persistent cyanosis when shunting blood from right to left • “Heart hump”, increased apex beat, systolic tremors with maximum in the second intercostal space to the left of the sternum • The borders of the heart are expanded to the left and right • Decrease in diastolic and increase in pulse blood pressure, strengthening of the apex beat, strengthening of both heart sounds (the loudness of the second sound above the PA correlates with the severity of pulmonary hypertension) • Rough machine systolic and diastolic murmur during II intercostal space to the left of the sternum, radiating into the interscapular space and to the great vessels • As pulmonary hypertension progresses and the discharge from left to right decreases, the murmur weakens and shortens until it disappears completely (at this stage, a Graham Still diastolic murmur may appear, arising due to relative insufficiency PA valve) followed by a re-increase when a right-to-left shunt occurs • Sometimes above the apex of the heart there is a murmur of relative stenosis or mitral valve insufficiency. Instrumental diagnostics • ECG: signs of hypertrophy and overload of the right and then left parts of the heart; rarely - blockade of the His bundle branches. • X-ray examination of the chest organs. Bulging of the PA arches, right and left ventricles. Enrichment of the pulmonary pattern, expansion and lack of structure of the roots of the lungs. Dilation of the ascending aorta. In adults, a calcified PDA can be visualized relatively rarely. • EchoCG. Hypertrophy and dilatation of the right and left ventricles. Visualization of the PDA, determination of its shape, length and internal diameters (to assess the prognosis and select the size of the endovascular occlusion device). In the Doppler mode, a specific form of shift of the Doppler frequency spectrum in the PA is identified, the degree of shunt and the ratio of pulmonary blood flow to systemic blood flow (Qp/Qs) are determined. • Catheterization of the left and right parts of the heart. The letter symptom is the passage of a catheter from the PA through the PDA into the descending aorta. An increase in blood oxygenation in the LA compared to the right ventricle by more than 2 volume percent. Tests with aminophylline and oxygen inhalation are performed to determine the prognosis regarding the reversibility of pulmonary hypertension. • Ascending aortography. Receipt of contrast agent from the ascending aorta into the PA. Diagnosis of concomitant coarctation of the aorta. Drug therapy. Before closing the PDA, prophylaxis against bacterial endocarditis is necessary. The use of indomethacin is indicated for narrow PDAs identified during the neonatal period, and is contraindicated in renal failure. Intravenous administration of indomethacin is recommended: • less than 2 days: initial dose of 200 mcg/kg; then 2 doses of 100 mcg/kg with an interval of 12–24 hours; • 2–7 days: initial dose 200 mcg/kg; then 2 doses of 200 mcg/kg with an interval of 12–24 hours; • more than 7 days: initial dose 200 mcg/kg; then 2 doses of 250 mcg/kg with an interval of 12–24 hours.
Surgical treatment Indications • Failure of conservative therapy for 5 days or more, contraindications to the use of NSAIDs • Decompensation of circulatory failure • PDA of medium or large diameter in all children under 1 year of age. Contraindications • Severe concomitant pathology that threatens the patient's life • Terminal stage of circulatory failure • Irreversible pulmonary hypertension. Methods of surgical treatment . In most cases, endovascular closure of the duct using occlusion devices (Gianturco coils, Cook coils, or umbrella devices) is feasible. If the duct is very wide or endovascular correction is unsuccessful, an open operation is performed: ligation or (less often) intersection of the PDA, followed by suturing of both ends. Thoracoscopic clipping of a PDA has no advantages over endovascular and open interventions, so it is rarely performed. Specific postoperative complications : injury to the left recurrent laryngeal nerve, bleeding, deformation of the aorta with the formation of coarctation, residual discharge of blood through the duct due to inadequate correction.
Forecast. A narrow PDA generally does not affect life expectancy, but increases the risk of infective endocarditis. Medium and wide PDAs almost never close on their own; spontaneous closure after 3 months is rare. The effectiveness of conservative treatment of narrow PDA reaches 90%. With PDA, mortality during the first year of life is 20%. Eisenmenger syndrome in older children is observed in 14% of cases, infective endocarditis and endarteritis - in 9% of cases. Duct aneurysm and its ruptures are isolated cases. The average life expectancy for medium PDA is 40 years, for wide PDA it is 25 years. Postoperative mortality is 3%. Clinical rehabilitation, depending on hemodynamic disorders, takes place over 1–5 years. Pregnancy. In women with a small or medium-sized PDA and left-to-right shunting, an uncomplicated course of physiological pregnancy can be expected. Women with high pulmonary resistance and a right-to-left shunt have an increased risk of complications. Synonyms: Open botal duct; Patent ductus arteriosus; Non-closure of the ductus botallus. Abbreviations. PDA - patent ductus arteriosus. PA - pulmonary artery.
ICD-10 • P29.3 Persistent fetal circulation in the newborn • Q21.4 Defect of the septum between the aorta and pulmonary artery • Q25.0 Patent ductus arteriosus
Prevention and prognosis
Since the pathology is congenital, there are no special methods of prevention. The risk of this defect can be reduced by ensuring normal conditions for pregnancy and fetal development.
The prognosis for this disease is different. If left untreated, the disease transforms from a white to a blue type of defect (with blood discharge in the direction from right to left). For a favorable prognosis, early diagnosis of patent ductus arteriosus is important.
If a child was born prematurely, then the presence of a patent ductus arteriosus is considered normal.
Normal physiology
The main task of the ductus arteriosus, along with the functioning foramen ovale, in the fetal period is to redirect blood flow from the right to the left, bypassing the lungs, while only 5-10% of cardiac output passes through the pulmonary circle [9]. The importance of a patent ductus arteriosus is emphasized by the fact that premature closure can lead to right ventricular failure and fetal hydrops.
The open state of the ductus arteriosus in the embryo is maintained by many factors, the most important of which are the relatively low oxygen tension and cyclooxygenase-dependent products of arachidonic acid metabolism (primarily prostaglandin PGE2 and prostacyclin PGI2), which are produced by the placenta. After birth, a sharp increase in oxygen tension inhibits potassium duct channels located in smooth muscle cells, resulting in an influx of calcium and compression of the vessel. Levels of PGE2 and PGI2, which are metabolized in the functioning lungs rather than entering the bloodstream as a result of the separation of the placenta, also fall.
Thus, the smooth muscle fibers in the duct contract, which leads to thickening of the walls and obliteration of the lumen. Functional closure of the duct usually occurs 24-48 hours after birth. Over the next 2-3 weeks, proliferation of the endothelium in the lumen is observed, which, together with destruction of the intima, leads to fibrosis and persistent obliteration of the duct with the formation of ligamentum arteriosum.
Epidemiology and incidence
The factors responsible for the persistence of a patent duct after birth are not fully understood. It is clear that preterm birth definitely increases the incidence of PDA. and this is due to physiological factors associated with prematurity rather than as a result of congenital ductal pathology [9]. In full-term infants, the incidence of PDA is about one case per 2 thousand births, which accounts for 5-10% of all congenital heart defects [9]. Women are more susceptible to the disease with a ratio to men of 2:1.
There is evidence that genetic factors may play an important role in many patients with PDA. Thus, PDA occurs with increased frequency in several genetic syndromes, including chromosomal abnormalities such as trisomy 21 and 4p syndrome, as well as gene mutations associated with Carpenter and Holt-Oram syndromes. In addition, the incidence may be influenced by external factors, such as prenatal rubella virus infection in the first trimester of pregnancy, especially in the first four weeks.
Clinical course and diagnosis
Clinical manifestations of PDA depend on the size of the shunt and can vary from complete absence of symptoms to the formation of severe heart failure in Eisenmenger syndrome. On physical examination, a characteristic feature of a PDA is a continuous systole-diastolic murmur along the left sternal border, often described as machine-like. The murmur is often accompanied by trembling of the chest and may be carried to the back.
Several ECG variants for PDA have been described [1]. ECG changes depend on the magnitude of the shunt from left to right, so the study can be either normal, with a small shunt, or sinus tachycardia or atrial fibrillation, LV hypertrophy and left atrial dilatation can be observed. In patients with a large PDA, biventricular hypertrophy may be observed against the background of severe pulmonary hypertension.
Echocardiography (EchoCG) is the method of choice in diagnosing and assessing the severity of PDA. Characteristic of a PDA is the registration of retrograde flow in the trunk of the pulmonary artery in the long-axis parasternal access at the level of the aortic valve or the flow from the descending aorta into the pulmonary artery in the suprasternal access when recording spectral or color Doppler mapping (CDC).
CDC is the most sensitive technique, allowing the detection of even tiny ducts. Typically, the color flow shows a continuous flame-shaped stream from the orifice of the duct at the bifurcation of the pulmonary artery, closer to the left branch, directed along the anterolateral wall of the pulmonary artery trunk. Spectral Doppler in a patient with low pulmonary artery pressure shows continuous, systole-diastolic flow with the highest velocity in mid-systole and lowest in late diastole. The sound signal obtained on a Doppler study is very similar to the auscultatory signal. In Eisenmenger syndrome, that is, when the pressure in the pulmonary artery is comparable to the systemic one, the flow becomes bidirectional, from the aorta to the pulmonary artery in diastole and from the pulmonary artery to the aorta in systole [7].
In addition to the actual detection of a PDA, it is necessary to evaluate its hemodynamic consequences. Typically, the size of the heart chambers is assessed and LV systolic function is determined. In a patient with a small PDA, the dimensions of the cavities are usually normal, although slight dilatation of the left atrium and/or LV may be present. A patient with a moderate or large PDA exhibits left-sided enlargement. In patients with high pulmonary artery pressure and low flow through the PDA or with a right-to-left shunt, diagnosis of the defect can be difficult, since demonstrating flow on the central circulation becomes problematic, even if it is large. Indirect signs, such as a flat interventricular septum, unexplained severe right ventricular hypertrophy and high-velocity pulmonary regurgitation, should require a more thorough investigation to exclude PDA [9].
In addition to the mandatory registration of the PDA jet on spectral Doppler and color flow, it is necessary to evaluate the pressure in the right ventricle from the tricuspid regurgitation signal. It is also possible to determine the diastolic pressure in the pulmonary artery from the flow of pulmonary regurgitation and assess the degree of shunting from left to right by determining the stroke volume in the outflow tract of the left and right ventricles.
One of the interesting forms of the disease is the so-called silent or silent PDA. A silent PDA is regarded as a silent PDA if: a) there is no characteristic systolic-diastolic murmur; b) absence of evidence of pulmonary hypertension on clinical examination, ECG and EchoCG; c) in the presence of a characteristic flow during color circulation and spectral examination of the pulmonary artery with a relatively high speed in diastole.
Silent PDAs are a common form of the disease with a possible incidence of up to one case per 500 people [6, 9]. Thus, in one of the studies, 21 patients with a silent duct were described, and 6 of them had no noise at all, two had soft noises of another defect, and the remaining 13 had so-called innocent noises [6].
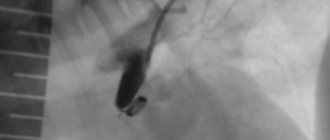
As an example, we give the following case. An 8-year-old girl was sent for echocardiography to the diagnostic department of the Krasnoyarsk Regional Hospital No. 2 due to suspected mitral valve prolapse. On auscultation, the patient heard a soft systolic murmur at Botkin's point. Clinically I felt satisfactory and led an active lifestyle. An echocardiogram examination of the heart cavity was within normal limits, “trivial” tricuspid regurgitation was detected, there were no signs of pulmonary hypertension, the inferior vena cava was of normal size and responded well to breathing. However, in the long-axis parasternal approach at the level of the aortic valve and in the suprasternal approach, an abnormal flow characteristic of a PDA was detected (Fig.)
.
Thus, based on clinical data and EchoCG signs, a silent PDA
.
Drawing. Color Doppler mapping. Left side. The suprasternal approach shows the blood flow from the descending aorta to the pulmonary artery (the descending aorta is presented in a cross section and is located above, the pulmonary artery is presented in a longitudinal section and is located below, the flow of the PDA is shown as a mosaic signal on the left side of the color scheme). Right part. Parasternal approach, short axis position at the level of the aortic valve. The pulmonary artery is located on the right side of the figure. The flow of the PDA is shown as a retrograde jet from the pulmonary artery bifurcation directed upward toward the pulmonary valve.
Hemodynamic consequences
The hemodynamic state of PDA in a normal heart is associated with the amount of left-to-right shunting. This depends on the size and shape of the duct, so traditionally there are large, moderate and small ducts, as well as on the pressure gradient between the aorta and the pulmonary artery. Left-to-right shunting through the PDA results in volume overload of the pulmonary circulation and left ventricle (LV). An increase in pulmonary blood flow leads to an increase in pulmonary fluid volume and a decrease in the elasticity of the lungs, which can affect lung function. In turn, an increase in venous return to the left sections leads to an increase in their end-diastolic pressure, dilatation and hypertrophy.
In the future, with large ducts and large discharge from left to right, hypertrophy of arteriolar copper, proliferation and fibrosis of the intima may be observed, which leads to a sharp increase in vascular resistance in the small circle. Eventually, the pulmonary artery pressure begins to approach or exceed the systemic pressure, and thus Eisenmenger syndrome is formed. In addition to Eisenmenger syndrome, other complications of PDA include the development of congestive heart failure, infectious endarteritis and ductal aneurysms, laryngeal nerve palsy, and rarely, dissection of the pulmonary artery and aorta.
Norilsk Interdistrict Children's Hospital
The article presents modern information about the pathogenesis and approaches to diagnosis and treatment of patent ductus arteriosus (PDA) in premature infants. A comparative description of markers of the hemodynamic significance of a PDA is given, and the role of the functioning of the duct in the formation of a number of diseases characteristic of premature newborns is shown. Studies are presented on various strategies for conservative and surgical treatment of PDA.
Improving the methods of caring for newborns in our country and the widespread use of surfactant preparations have not only significantly increased the survival rate of premature newborns, but also posed new problems for neonatologists. One of them is patent ductus arteriosus (PDA).
For a long time in our country there were no officially registered drugs for the medical correction of this condition; the possibility of surgical treatment of premature infants was also presented in a small part of neonatal intensive care units. Diagnostic approaches and indications for therapy were often borrowed from protocols for the management of full-term newborns with congenital heart defects (CHD).
Until the end of the 90s of the 20th century, there were isolated references in the domestic literature to the problem of PDA in premature infants [1-3]. As a result, until recently in Russia there were no unified diagnostic and therapeutic approaches to PDA in premature newborns. In the presented material, we would like to summarize international and domestic experience in improving knowledge about the physiology of persistent ductus arteriosus (DA) in premature newborns, modern approaches to the diagnosis and treatment of this condition. Historical reference. Fetal circulation was first described by Galen (130-200). In 1583, the Italian physician and anatomist Leonardo Botalio rediscovered and described the vessel connecting the aorta and pulmonary artery, and named it AP. The Basel specification of 1895 gave this vessel its name. The clinical diagnosis of PDA was first reported by Bernuts in 1847. In 1907, Munro spoke at a meeting of the Philadelphia Surgical Society with the idea of surgical treatment of PDA.
The world's first successful operation to close a PDA in 1938 was performed by general surgeon R. Gross on a 7-year-old patient. The first such successful operation in our country was performed in 1948 by A.N. Bakulev. In a premature infant weighing 1413 g, closure of the PDA was first performed in 1963. Thus, for 13 years, until 1976, when indomethacin was first used to close the PDA, surgical treatment remained the only treatment option for PDA in premature infants. Since 1995, in addition to indomethacin, ibuprofen has also been used in the world to close PDA, which has a number of advantages. In October 2008, the first drug for closing a PDA in premature infants, Pedea®, was registered in Russia.
Prevalence. PDA is one of the pathological conditions characteristic of very premature newborns, especially those suffering from respiratory distress syndrome (RDS). The incidence of PDA is inversely proportional to gestational age (GA) and body weight (BW) at birth. Thus, in newborns with a GA of less than 28 weeks and with a BW of less than 1000 g, the need for treatment of PDA is 55-70% [4]. According to Fanaroff A.A. et al. [5], the frequency of hemodynamically significant functioning AP (HSFAP) in newborns with very low body weight at birth (VLBW) ranges from 13% in children with a body weight of 1251-1500 g to 49% in newborns with a body weight of 501-750 g [5].
The diagnosis of a PDA is usually made if it does not close on its own by 72 hours of life [6, 7]. The AP is one of the main components of the fetal circulation - it is a vessel connecting the left pulmonary artery and the descending aorta. High pulmonary vascular resistance (due to constriction of the pulmonary arterioles) and low placental vascular resistance maintain the direction of blood flow from right to left through the AP and back to the placenta [8, 9].
Direction of blood shunting. Terminology issues. The question of the direction of blood flow through the AP is fundamental to determining the clinical significance of the shunt. There is a common misconception that, despite impaired closure of the pulmonary artery in premature infants after birth, the pressure in the pulmonary artery system is so high that in the first days of life there is little blood discharge through the vessel.
In fact, only a small proportion of premature newborns have pulmonary artery pressures that high. In the majority of premature infants, systemic arterial pressure significantly exceeds the pressure in the pulmonary artery, which determines the direction of blood shunting predominantly from left to right [10].
In foreign literature, the term PDA (“patent ductus arteriosus”) defines the shunting of blood from left to right - from the descending aorta to the pulmonary artery. Shunting of blood in the opposite direction - from the pulmonary artery to the aorta - is part of the persistent fetal circulation syndrome in conditions of persistent pulmonary hypertension.
In essence, this term determines the direction of blood flow, since normally a left-to-right shunt never occurs in the fetus. At the same time, according to ICD 10, the term “persistent fetal circulation in the newborn” is used to refer to “delayed closure of the ductus arteriosus in the newborn” (code P29.3).
Attention should be paid to the fact that two different pathologies requiring different treatment have the same code. In 2009, the Russian Association of Perinatal Medicine Specialists recommended expanding the list of diagnoses to a three-digit level - using code P29.3.1 for delayed closure of AP in a newborn and code P29.3.2 for persistent fetal circulation (persistent pulmonary hypertension) in a newborn.
To indicate persistent AP or congenital anomalies of the heart, the code “patent ductus arteriosus” Q25.0 is used in the section “Congenital anomalies (malformations) of the large arteries” [11]. Regulation of the tone of the AP wall. Antenatal functioning of the AP is achieved as a result of a balance between two groups of factors that promote the closure of the duct and maintain it open. The factors responsible for the antenatal increase in PDA tone have been little studied. These include the level of extracellular calcium.
It has been proven that the sensitivity of the smooth muscle wall of the PDA to the contractile influence of calcium is much higher than that of the walls of the aorta and pulmonary artery. Endothelin 1 also plays an important role in the formation of the tone of the AP wall [12, 13]. The factors that contribute to maintaining an open AP have been much better studied. First of all, this is high blood pressure in the lumen of the vessel, caused by high vascular resistance of the pulmonary arterioles [13]. The AP wall is sensitive not only to the action of prostaglandins (PGs) produced in the wall itself, but also to the level of circulating PGE2.
The main source of PGs is the placenta, and their catabolism occurs in lung tissue. Thus, in the fetus, under conditions of sharply reduced pulmonary blood flow, the prerequisites are created for a high concentration of PG in the blood [14]. The significant role of endogenous nitric oxide (NO), which is also produced in the wall of the AP and maintains it open, has been proven both clinically and experimentally [15, 16]. Carbon monoxide (CO) is also a vasodilator and is found in the endothelium and muscle wall of the AP. The amount of CO produced by the AP wall under normal conditions cannot significantly affect its tone, while at the same time, with an increase in CO synthesis, for example, with endotoxemia, its vasodilating effect may occur [14].
Like most smooth muscle vessels, under the influence of hypoxia in the AP there is a decrease in the tone of the muscle wall. The relatively low oxygen content in the fetal blood leads to the fact that the AP remains open [17]. The combination of intimal thickening with vascular constriction due to increased blood oxygen levels leads to functional closure of the AP after birth (usually within the first hours of life). Anatomical closure with differentiation and apoptosis of smooth muscle cells subsequently leads to the formation of an arterial ligament (by the 3-4th week of life) [18].
AP closure after birth. The starting point for the restructuring of the blood circulation of a newborn baby is the ligation of the umbilical cord and the first breath. The cessation of umbilical blood flow (connection to the placenta) leads to a sharp decrease in circulating PG levels and an increase in systemic blood pressure. Filling the lungs with air and the onset of gas exchange leads to a decrease in mechanical compression of the pulmonary vessels by pulmonary tissue, an increase in blood oxygen tension (PaO2), which leads to a sharp increase in pulmonary blood flow and a decrease in pulmonary vascular resistance.
Thus, the direction of flow through the AP changes to left-right, and then, under conditions of low pulmonary resistance, the blood flow through the AP stops. The postnatal increase in the level of partial pressure of blood oxygen (PaO2) also plays a significant role. Cytochrome P450, located in the membrane of the muscular choroid cells, plays the role of a receptor in the vasoconstrictor effect of oxygen on the wall of the AP. Oxygen blocks K+ channels. This leads to membrane depolarization and an increase in the content of intracellular calcium in the muscle wall of the vessel, which leads to an increase in its tone [13].
Immediately after birth, the AP spasms, but does not immediately close. Most PDAs recorded in the first week of life in full-term newborns close spontaneously [19]. At the same time, in premature newborns, especially children with extremely low body weight at birth (ELBW), there is often a violation of the mechanisms of AP closure. Even if functional closure of the vessel occurs after birth, the stage of deep ischemia of the muscle wall is rarely reached, which creates the preconditions for repeated openings of the artery.
In addition, there is a clear connection between neonatal immaturity, RDS, infectious diseases and the risk of persistent PDA [13, 19, 20]. A number of mechanisms of the immature child described above are aimed at maintaining the AP open even after birth. Internal factors include immature muscularis propria and vasodilating substances produced by the AP wall (PG, endogenous NO). External factors include low levels of cortisol in premature newborns (cortisol helps reduce the synthesis of PGs and reduces the sensitivity of the AP wall to their effects), high levels of circulating PGs [21].
One of the factors contributing to the release of PG into the blood is, for example, artificial pulmonary ventilation (ALV), since lung tissue is rich in arachidonic acid, a precursor of PG [22]. By the age of 7 days of life, the level of PG in the blood tends to decrease, which explains the decrease in the effectiveness of cyclooxygenase inhibitors in the treatment of PDA. Later (over 7 days), re-opening of the AP is almost always caused by an infectious process due to the release of pro-inflammatory cytokines.
The most important role in this is played by tumor necrosis factor a (TNFa). This inflammatory mediator, the level of which is significantly increased in newborns with late onset of AP, triggers a metabolic cascade, at the end of which are, in particular, endogenous NO and PG [23]. The timing of AP closure in preterm infants varies significantly.
A number of authors note that the faster the vessel constriction occurs in the first hours after birth, the more likely spontaneous closure of the PDA is. The exception is very premature newborns (GA<27 weeks) [13]
PDA and BPD. Most newborns with PDA require long-term mechanical ventilation and oxygen supplementation. In addition, the incidence of PDA increases with decreasing GV. Thus, the risk factors for the formation of BPD and the therapeutic aspects of PDA treatment coincide. In addition, a number of studies have proven that the functioning of the PDA, independently and especially in combination with an infectious process, is a risk factor for the formation of BPD [23, 32].
Repeated late (at the age of more than 7 days) opening of the AP and its long-term functioning significantly more often lead to the formation of BPD than the so-called “early” PDA, registered in the first week of life [33]. A number of authors draw attention to the fact that, despite the obviousness of the statement that the functioning of the PDA is a risk factor for the formation of BPD, the prophylactic use of non-steroidal anti-inflammatory drugs to close the PDA does not reduce the incidence of BPD development [34].
This may probably be due to the fact that the formation of BPD is influenced to a greater extent by the duration of the functioning of the PDA, and not by the fact of its presence itself. In addition, it has been suggested that the functioning of the PDA may be a marker of immaturity, including of lung tissue.
Diagnosis of PDA. Clinical signs (systolic murmur, increased cardiac impulse, racing pulse) in premature newborns have low sensitivity in diagnosing PDA. Their importance increases after 4 days of life. In this case, the hemodynamic significance precedes the appearance of clinical symptoms by an average of 2 days (from 1 to 4 days) [40].
Another classic sign of PDA is considered to be a large systolic-diastolic difference. However, there was no difference in blood pressure in preterm infants with and without PDA during the first week of life. PDA has been proven to have a negative effect on both systolic and diastolic blood pressure. As a result, children with PDA had significantly lower mean arterial pressure, but there was no difference in pulse pressure [41].
Accurate diagnosis of the presence and hemodynamic significance of AP is possible only with echocardiography. The diagnostic value of determining the diameter of the AP and the direction of shunting along it is beyond doubt, in contrast to other signs of the hemodynamic significance of the AP, the reliability of which is widely discussed in the medical literature. The study by Evanse et al. [42] included newborns with a birth weight less than 1500 g with a minimal shunt through the foramen ovale.
The criterion that had the greatest correlation with the Qp:Qs indicator (the ratio of pulmonary to systemic blood flow) was the diameter of the PDA. When examined during the first week of life, the diameter of the artery was less than 1.5 mm and usually had no hemodynamic significance; when the diameter increased to more than 1.5 mm, the shunt became hemodynamically significant. When the AP diameter was more than 2 mm, the Qp:Qs ratio was more than 2:1. Another reliable indicator is the diastolic flow in the postductal region of the descending aorta.
Under normal conditions, the blood flow in this section of the aorta is unidirectional, but in the presence of a functioning shunt, the blood flow in diastole is directed to the aorta and Doppler sonography records the blood flow first tending to the isoline and then retrograde. Retrograde flow is associated with a Qp:Qs of 1.6. Thus, pulmonary blood flow is 60% greater than systemic blood flow [19, 42]. The same thing happens on the reverse side of the shunt, where the diastolic flow in the left branch of the pulmonary artery increases, which can also be an indicator of the hemodynamic significance of the shunt [43].
In addition to the above criteria for the hemodynamic significance of blood shunting through the PDA, the ratio of the diameter of the left atrium to the diameter of the aortic root (LA/Ao), the ratio of the end-diastolic size of the left ventricle to the aortic root, as well as an increase in indices of vascular resistance in cerebral vessels are also used [44]. One of the new echocardiographic criteria that has demonstrated high (90%) sensitivity and specificity is the ratio of left ventricular output to superior vena cava flow (LVO/SVC) [45].
Among the new methods for objective assessment of the hemodynamic significance of the shunt through the AP, the study of type B natriuretic hormone (BNUP) [46-48], cardiotroponin T (cTpT) [49, 50] are discussed.
Management of newborns with hemodynamically significant PDA. There are three ways to manage preterm infants with PDA: conservative management, surgical treatment, and medical closure with nonsteroidal anti-inflammatory drugs (NSAIDs).
Recently, the conservative management of newborns with PDA has been actively discussed by the medical community. It is suggested that there is a certain category of relatively mature newborns for whom the functioning of the PDA is not dangerous in relation to the development of the pathological conditions described above. According to the literature [51, 53], spontaneous closure of the AP is described in 86% of newborns (average GA = 28 weeks, BW = 998 g) discharged from the hospital with a PDA within 11 months.
Surgery. There is currently no evidence of any advantages of surgical treatment of PDA over medication. In 1983, a multicenter randomized trial was conducted comparing the outcomes of newborns initially operated on in the first days of life for the functioning of the PDA and treated with indomethacin.
A high incidence of pneumothorax and retinopathy of prematurity in operated children was revealed. However, no differences were found in other outcomes [54]. The work of Cassady et al. [55] in 1989 showed a lower incidence of enterocolitis in a group of newborns who underwent early prophylactic ligation of the PDA; no differences were noted in other outcomes.
The surgical method of correction is recognized as associated with a large number of complications. In a randomized trial, Kabra et al. [56] found that surgical correction increases the risk of poor neurological outcomes and significantly increases the risk of severe retinopathy of prematurity.
In another study, Chorne N. et al. [57] did not reveal the effect of surgical correction on increasing the risk of developing neurological complications, but it was noted that surgical correction is an independent risk factor for the development of BPD. In most studies assessing the negative impact of surgical treatment of PDA, there is a longer functioning of the PDA in the group of children who underwent surgical correction.
Currently, in most clinics, surgical correction of a PDA is performed when two courses of drug correction are ineffective or the re-opening of the PDA is late. The question of how much the operation protects the child from further complications characteristic of the functioning of the PDA remains controversial [38]. In accordance with the recommendations of the Russian Association of Perinatal Medicine Specialists, surgical correction of HFAP is performed only in newborns dependent on mechanical ventilation when two courses of drug therapy with COX inhibitors are ineffective, there are contraindications for their use, and when the newborn is more than 7 days old [11].
Drug therapy for HZFAP. A large number of studies have compared different drug therapy strategies for HSFAP. The most complete comparative description of these studies is presented in the work of David B. Knight [24].
Indomethacyniibuprofen is used as COX inhibitors. Both drugs are equally effective in closing the AP. The use of ibuprofen for AP closure was developed as an alternative to the use of indomethacin. Ibuprofen has significantly less effect on renal, mesenteric and cerebral blood flow [58, 59]. In a comparative study by Van Overmeire et al. [60] both drugs were equally effective in closing a PDA; there was no difference in the frequency of the need for a second course of therapy or surgical correction.
There was a lower incidence of oliguria in neonates receiving ibuprofen. A meta-analysis of 16 studies (876 VLBW children treated with ibuprofen or indomethacin for the treatment of PDA) showed no significant difference in the rate of treatment failure, the need for surgical correction and mortality.
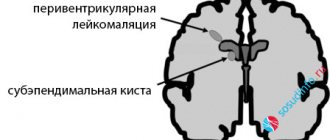
There was also no significant difference in worsening outcomes - BPD, severe IVH, periventricular leukomalacia (PVL), NEC, intestinal perforation, retinopathy of prematurity. In 6 studies (336 children), a significantly lower blood creatinine level was noted, and in 3 studies (358 children), a significantly lower incidence of oliguria was noted in newborns receiving ibuprofen [61]. The high cost of the intravenous form of ibuprofen in comparison with indomethacin and the unavailability of this drug in a number of countries have led to studies of the effectiveness and safety of oral ibuprofen.
Currently, there are 7 small randomized studies devoted to this problem (the total number of children included in the studies is 208) and confirming the effectiveness of the drug in closing PDA [62, 63]. However, there are several reports of serious complications associated with the administration of ibuprofen by mouth - the development of acute renal failure [64] and intestinal perforation [65], which does not allow us to recommend this method of treatment for PDA.
One of the most controversial issues in the management of children with PDA remains the timing of initiation of NSAID treatment. It should be immediately noted that the drug registered in Russia - ibuprofen solution for intravenous administration Pedea® - is not recommended for prophylactic use.
In a study by van Overmeire et al. [66] compared therapeutic administration of indomethacin on days 3 and 7. In the day 3 group, there were significantly more side effects associated with indomethacin administration, but no benefit in terms of respiratory outcomes or mortality. A meta-analysis found that early administration of indomethacin was associated with a lower incidence of subsequent PDA closure, but no difference in outcomes, including BPD or mortality [26].
Based on the above, if the protocol provides for later (7th day) administration of the drug, one can expect that the PDA will close on its own, but, on the other hand, taking into account the early hemodynamic complications (up to 3-5 days) PDA - pulmonary hemorrhages, NEC, early IVH, possibly late administration, may significantly worsen outcomes in VLBW infants. Recommendations for the management of PDA in premature infants vary significantly across countries and clinics.
A promising option for the management of children with PDA, recommended in the work of the Australian researcher N. Evans [19]. The Department of Neonatal Medicine Royal Prince Alfred Hospital protocol provides for the prescription of NSAIDs based on dynamic monitoring of the diameter of the PDA. All children at risk undergo an echocardiographic examination at the age of 3-6 hours of life. If the diameter of the AP is more than the median of 2.0 mm at the age of 3 hours, the child receives the first injection of the drug [19].
Unfortunately, in modern Russian conditions, in the absence of the possibility of promptly performing echocardiography in newborns in the first hours of life, such tactics are still unacceptable in most clinics. The problem of timely diagnosis and treatment of HFAP in newborns with VLBW and ELBW is an integral part of improving the treatment of very premature newborns, along with the use of surfactant preparations, the introduction of new methods of respiratory therapy, optimization of enteral and parenteral nutrition and other components of the management of this group of patients.
International experience in the use of drugs for the treatment of PDA, accumulated over 30 years, and Russian clinical experience in the future should significantly improve outcomes in newborns with VLBW and ELBW.