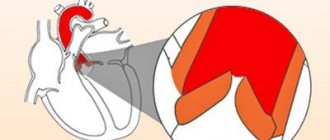
An important anatomical component of the heart is the valve apparatus. Without it, blood would not be able to move in one direction, which is why it is extremely important that all valves are in good condition and functioning correctly. Variants of the norm and physiological characteristics of the heart valve apparatus will be considered.
Heart valves are necessary for the portioned ejection of blood during contraction of the heart. Their main function is to prevent backflow of blood (regurgitation) and ensure that it always moves in the same direction through the heart. The closure of heart valves can usually be heard with a stethoscope, which can be used for the initial diagnosis of valvular conditions.
Video: Structure of the human heart, blood circulation circles
Description
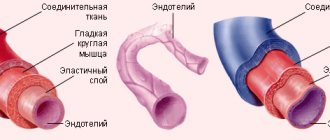
All heart valves are covered with endothelium. The three layers that form the basis of the valve apparatus have specific characteristics and are called fibrosa, spongiosa and ventricularis. During heart contractions, spongiosa, rich in glycosaminoglycans, facilitates the process of rearrangement of collagen and elastic fibers.
Vacular interstitial cells (VICs) are abundant in all layers of heart valves and contain a variety of dynamically targeted components. The regulation of collagen and other structural components is provided by enzymes synthesized by VIC. The integrity of valve tissue is maintained by the interaction of valve endothelial cells (VECs) with the VIC. Changes and remodeling of the valvular interstitial and endothelial structure contribute to disruption of the valve properties, and subsequently valve function.
Basics of proper operation of the valve apparatus:
- The valves are properly formed and flexible.
- The valves open completely, allowing the required amount of blood to pass freely through the opening.
- The valves close tightly, then blood does not flow back
Types of valves used in prosthetics
Biological valves
They can be made from animal or human tissues (heterografts, homografts, autografts). Biological valves may contain some artificial components to provide valve support and placement. The main advantage of such a valve is that there is no need for lifelong anticoagulant therapy (constant strict use of drugs that significantly thin the blood and require constant blood tests), and the main disadvantage is its limited service life (10-15 years).
Mechanical valves
They consist entirely of mechanical elements (titanium and pyrolytic carbon) and are designed to replace the patient's own valve functions. The mechanical valve is very reliable and durable, designed for many years of full-fledged operation, which is the main advantage, but requires the patient to constantly take anticoagulants.
The Center for Cardiac Surgery and Cardiology provides assistance to patients with valvular pathology of varying complexity, including one-, two- and three-valve replacement with biological and mechanical prostheses of Russian and foreign production, elimination of valvular insufficiency using a variety of plastics (plasty on a support ring, tri- and quadriangular resection leaflets with annuloplasty according to De Vega, Batista) of the valve apparatus, restoration of the chordal apparatus by prosthetics of the mitral valve chords, etc. The main direction in surgery of valve pathology is aimed at increasing the number of reconstructive operations on the valve apparatus and reducing valve replacement.
The Clinic offers surgical treatment of heart valves in combination with other diseases, such as coronary heart disease, atrial fibrillation (atrial fibrillation).
Aortic valve
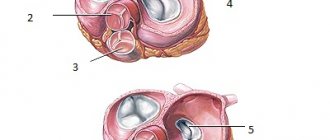
The tricuspid valve, located at the mouth of the aorta, separates the cavity of the left ventricle from the aorta. Behind the three semilunar cusps (right coronary, left coronary and posterior non-coronary) of the aortic valve are dilated pockets of the aortic ostium, called sinuses of Valsalva. The right coronary artery arises from the right coronary sinus, and the left coronary artery arises from the left coronary sinus. The area where all three valves meet is called the commissure.
The opening and closing of the aortic valve is a passive pressure-controlled mechanism, unlike the mitral valve.
The tissue of the aortic valves is stretched as a result of counteraction during diastole, and elastin elongates and stretches. Therefore, normally the aortic valve leaflets are quite flexible and durable, able to withstand systemic pressure. During the systole phase, the release of elastin ensures relaxation and shortening of the leaflet. Optimal valve function requires perfect alignment of the three return points.
Associated diseases: aortic regurgitation (also called aortic regurgitation), aortic stenosis.
4.Diagnosis and treatment of the disease
Diagnosis
is established by a cardiologist based on complaints, anamnesis, results of auscultation, percussion and additional studies (ECG, ultrasound, etc.).
Treatment
can range from recommendations to follow certain rules and restrictions in daily life - and these recommendations should be taken seriously - to surgery. Over the past decades, cardiac surgery has accumulated a rich arsenal of methods for effective correction, implantation, and surgical reconstruction, which makes it possible to successfully cope with many serious and dangerous problems in the functioning of the heart muscle and its valves.
Mitral valve
The mitral valve was named after the mitre by Andreas Vesalius (De Humani Corporis Fabrica, 1543). This valve is located at the junction of the left atrium and the left ventricle. Its structure consists of five functional components:
- doors;
- annular space;
- chordae tendineae;
- papillary muscles;
- nearby myocardium.
The annulus fibrosus is an area of connective tissue containing discontinuous fibrous and muscle fibers that connect to the left atrium and ventricle. The anterior valve covers about one-third of the primary fibrous anterior part of the ring. Part of the anterior leaflet of the mitral valve is located in close proximity to the annular opening of the aortic valve. The ventricular, posterior, leaflet is attached to the posterior muscular half and two-thirds of the annular space. Due to the asymmetrical leaflets, the opening of the mitral valve is shaped like a funnel.
Chords from both the anterior and posterior papillary muscles are attached to each valve. The papillary muscles contract and extend the chordae during systole, which in turn promotes the closure of the mitral valve leaflets.
The mitral valve complex is separately distinguished, including the mitral valve and the left atrioventricular myocardium, endocardium and part of the aorta. This formation promotes the outflow of blood from the left ventricle. The forced passage of blood through the valve, as well as its tight closure during systole, is ensured by the coordination of the actions of the mitral-valve complex.
Associated diseases: mitral valve prolapse, mitral valve regurgitation, mitral valve stenosis.
Artificial heart valves
Recommendations for patients with a prosthetic heart valve 1.6 MB
Artificial heart valve: 2 main types
If any of the 4 heart valves malfunction - they are narrowed (stenosis) or excessively dilated (insufficiency) - it is possible to replace them or reconstruct them using artificial analogues. An artificial heart valve is a prosthesis that provides the required direction of blood flow by intermittently closing the mouths of venous and arterial vessels. The main indication for prosthetics is gross changes in the valve leaflets, leading to severe circulatory disorders.
There are two main types of artificial heart valves: mechanical and biological models, each of which has its own characteristics, advantages and disadvantages.
1. Butchart EG et al. Recommendations for the management of patients after heart valve surgery. European Heart Journal. 2005: 26(22); 2465-2471.
Figure 1. Two main types of artificial valves
Mechanical heart valve or biological prosthesis?
The mechanical heart valve is reliable, lasts a long time and does not need to be replaced, but requires constant use of special medications that reduce blood clotting.
2. Bonow RO, Carabello BA, Kanu C. et al.; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Society of Cardiovascular Anesthesiologists; Society for Cardiovascular Angiography and Interventions; Society of Thoracic Surgeons. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease) : developed in collaboration with the Society of Cardiovascular Anesthesiologists: endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Circulation 2006; 114(5):e84-231; J Am Coll Cardiol 2006; 48(3):e1-148.
Biological valves may gradually deteriorate. Their service life largely depends on the age of the patient and concomitant diseases. With age, the process of destruction of biological valves slows down significantly.
The decision about which valve is most optimal should be made before surgery during a mandatory conversation between the surgeon and the patient.
Life with an artificial heart valve
People with prosthetic heart valves are at very high risk of thromboembolic complications. The fight against thrombosis is the basis of the management strategy for such patients, and it is its success that largely determines the prognosis for the patient.
The risk of thromboembolic complications is reduced with the use of biological valve prostheses, but they have their disadvantages. They are implanted infrequently and mainly in older people.
Living with an artificial heart valve requires a number of restrictions. The majority of patients with prosthetic valves are those with mechanical prostheses, who belong to a group at high risk of developing thrombotic complications. The patient is forced to constantly take antithrombotic drugs, in the vast majority of cases - indirect anticoagulants (warfarin). Almost all patients with mechanical heart valves should take them. The choice of a bioprosthesis also does not exclude the need to take warfarin, especially in patients with atrial fibrillation. To avoid dangerous bleeding, patients chronically taking warfarin should avoid daily activities and entertainment associated with an increased risk of injury (contact sports, working with cutting objects, or a high risk of falls, even from height).
The most important aspects of medical monitoring of a patient with an artificial heart valve today include:
- blood clotting control;
- active prevention of thromboembolic complications using anticoagulants (most often warfarin).
3. Bonow RO, Carabello BA, Chatterjee K. et al.; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease). Endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation 2008;118(15):e523-661; J Am Coll Cardiol 2008; 52(13):e1-142.
It is important to note that European and American experts now consider the levels of antithrombotic therapy previously recommended for most patients to be too intense. Modern approaches to risk assessment make it possible to identify subgroups of people with the highest risk of thromboembolic complications and active antithrombotic therapy. For other patients with prosthetic heart valves, less aggressive antithrombotic therapy may be more effective.
Prevention of thrombosis in patients with mechanical heart valves
Prevention of thrombosis in patients with a mechanical heart valve requires lifelong antithrombotic therapy.
The intensity of warfarin therapy depends on the location of the prosthesis and its type. For example, in accordance with the ACC/AHA (2008) recommendations, a mechanical aortic valve prosthesis requires maintaining an INR in the range of 2.0-3.0 when using double-leaf (bicuspid) prostheses, as well as the Medtronic Hall valve (one of the most popular single-leaf artificial valves in the world). valves), or in the range of 2.5-3.5 for all other disc valves, as well as for the Starr-Edwards ball valve.
4. Salem DN, O'Gara PT, Madias C., Pauker SG; American College of Chest Physicians. Valvular and structural heart disease: American College of Chest Physicians Evidence
Mechanical mitral valve prosthesis requires maintaining the INR within 2.5-3.5 for all types of valves.
Table 1. Recommended INR value for mechanical heart valves
| Heart valve position | Risk factors for TE complications | |
| none | present | |
| Aortic | 2,0-3,0 | 2,5-3,5 |
| Mitral | 2,5-3,5 | 3,0-4,0 |
5. Methodological recommendations were reviewed and recommended by the scientific council of the Federal State Budgetary Institution “Research Institute for Complex Problems of Cardiovascular Diseases” of the Siberian Branch of the Russian Academy of Medical Sciences on July 1, 2011, updated on January 14, 2014.
However, even with recommended antithrombotic therapy, the risk of thromboembolic complications in patients undergoing heart valve replacement remains at 1-2%. The results of most clinical studies indicate that the risk of thrombosis is higher in patients with mitral valve prostheses (compared to aortic valve prostheses). If for patients with artificial aortic valves a less intensive anticoagulant regimen is possible (with a target INR of 2.0-3.0), then in the case of a mechanical mitral valve prosthesis, the anticoagulant regimen should be quite intensive (with a target INR of 2.5-3 ,5).
6. Vahanian A., Baumgartner H., Bax J. et al.; Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology; ESC Committee for Practice Guidelines. Guidelines on the management of valvular heart disease: The Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology. Eur Heart J 2007; 28 (2): 230-68.
Regardless of the type of artificial valve used, the risk of thrombosis is highest in the first few months after surgery - until the completion of epithelization processes at the site of implantation of the prosthesis. American experts consider it advisable to keep the INR within 2.5-3.5 in the first 3 months. after surgery, even for patients with an artificial aortic valve.
In addition, keeping the INR within a more stringent range (2.5-3.5) is recommended by the ACC/AHA in the presence of high-risk factors for thromboembolism, regardless of the type of prosthesis and its location. Such factors include atrial fibrillation, a history of thromboembolism, left ventricular (LV) dysfunction, and a hypercoagulable state.
Currently, there are portable devices for self-determination of INR (similar to systems for monitoring sugar levels in patients with diabetes), which help maintain INR levels in the required range. Among them, Coagucheck XS has proven itself for independent testing and immediate receipt of PTT/INR results. The device allows you to get accurate results in less than a minute, using only 8 µl (one drop of blood).
However, regardless of the chosen antithrombotic treatment strategy after heart valve replacement, regular patient monitoring, education, and close collaboration with the treating physician remain essential.
7. Butchart EG Antithrombotic management in patients with prosthetic valves: a comparison of American and European guidelines. Heart 2009;95: 430 436.
This allows for timely adjustment of drug doses, as well as changes in their thrombolytic activity, depending on the nutritional characteristics, state of the patient’s liver and kidney function.
Prevention of thrombosis in patients with bioprosthetic valves
In patients with bioprosthetic valves, less aggressive anticoagulant therapy is indicated, since in most studies the risk of thromboembolic complications in such patients, even in the absence of anticoagulant therapy, averaged only 0.7%.
According to American experts, the addition of warfarin may be useful in cases of increased risk of thromboembolism, but is not routinely recommended for all patients. When using warfarin, the INR should be kept within 2.0-3.0 if the aortic valve is replaced, and 2.5-3.5 if the mitral valve is replaced.
The use of warfarin with a target INR of 2.0-3.0 may also be appropriate in the first 3 months. after surgery and in patients with a mitral or aortic valve prosthesis without risk factors, given the increased tendency to thrombus formation in the early stages after valve replacement. Patients with a mitral valve prosthesis benefit particularly from this strategy.
Table 2. Recommended INR value for biological heart valves
| Heart valve position | Risk factors for TE complications | |
| none | present | |
| Aortic | 2,0-2,5 | 2,5-3,0 |
| Mitral | 2,5-3,0 | 3,0-3,5 |
| Tricuspid | 2,5-3,0 | 3,0-3,5 |
However, European ESC experts believe that there is currently insufficient evidence to support the need for long-term antithrombotic therapy in patients with bioprosthetic heart valves, unless these patients have any additional risk factors.
European guidelines recommend the use of warfarin in such patients only for the first 3 months. after surgery (target INR - 2.5).
Long-term (lifelong) anticoagulation therapy in patients with bioprosthetic valves may only be appropriate in the presence of high-risk factors (eg, atrial fibrillation; to a lesser extent, heart failure with LVEF <30% may be a risk factor), according to ESC guidelines6.
Thus, for patients with bioprosthetic heart valves, European experts recommend a more cautious tactic of antithrombotic therapy, while American experts consider a more aggressive approach justified. At the same time, in the United States, there is a more widespread tendency to minimize the patient’s time in hospital and the cost of his treatment, so American doctors prefer to prescribe acetylsalicylic acid to patients with bioprostheses. In Europe, they are still inclined to keep the patient in the hospital longer, if necessary, and to use warfarin in this category of patients, which is more demanding in monitoring blood coagulation parameters.
One of the most significant problems in the management of such patients in domestic healthcare settings is the inability to adequately control blood coagulation parameters against the background of constant use of anticoagulants.
It is the INR indicator that is recommended by all international guidelines as necessary to ensure the safety and effectiveness of therapy.
Recommendations for patients with a prosthetic heart valve 1.6 MB
Pulmonary valve
Also known as the pulmonary valve. The structure of the pulmonary valve is similar to that of the aortic valve. The valves have a semilunar shape; normally there are three of them (anterior, left and right). Similar to the valves, the sinuses are called sinuses, which are united with the pulmonary trunk through an arched ring (sinotobular junction). Like other valves, the pulmonary valve also has a fibrous ring and a commissure.
Associated diseases: pulmonary valve stenosis, pulmonary valve regurgitation.
Pulmonary snort
In the protected state of the tricuspid snort, the only route for blood is through the pulmonary trunk. This valve, in accordance with anatomy, is located at the inlet. Its structure is such that when pressure increases, it opens and allows blood flow into the arteries. Under the influence of the return flow, in a relaxed state of the ventricle, it is blocked, identical to the aortic one, protecting the pulmonary trunk from the reverse flow of blood.
The right chamber is a system in which the pressure is reduced. Therefore, the structure of the snort here is softer in comparison with the aortic one. When listening to a person in good health, the doctor hears the pulmonary and aortic valves of the heart.
Tricuspid valve
Also known as the tricuspid valve. Located in the right half of the heart at the junction of the atrium and ventricle. Consists of 3 valves, chordae tendons (anterior, posterior) and the often identified third papillary muscle. The tricuspid valve does not have a clearly defined collagen ring. The three flaps are attached to an elliptical shaped fibrous ring. The direct attachment of the cuspid septum is a hallmark of the tricuspid valve. Prominent papillary muscles support the valves in the commissures.
Normal valve function requires structural integrity and coordinated interactions between multiple anatomical components. Various pathophysiological mechanisms can cause heart valve disease.
Associated diseases: tricuspid atresia, tricuspid regurgitation, tricuspid stenosis.
2.Danger of valve pathology
Any pathology in the mechanics of one or more heart valves inevitably affects the functioning of the myocardium as a whole, and therefore the entire life support system - no matter how stable it may be. The most common negative effect in this sense is that the heart muscle, automatically compensating for blood deficiency and working with constant overload, gradually hypertrophies
– increases in volume, stretches.
As a result, the so-called heart failure to varying (sometimes life-threatening) degrees. Arrhythmia, a tendency to thrombus formation due to blood stagnation, erosion,
etc.
may occur shortness of breath
, i.e. feeling of lack of air. Actually, this is a deficiency of oxygen in the blood, which is not oxygenated or purified properly.
Visit our Cardiology page
Operation of the valve apparatus
In normal condition, the valves function in strict order, which allows the chambers of the heart to contract correctly and eject blood in the required volume. There are four main stages in the operation of the valve apparatus:
1. The atrioventricular valves (mitral and tricuspid) open, as a result of which blood rushes from the upper parts of the heart to the lower ones.
2. When the ventricles are filled, the pressure in their cavity increases, as a result of which the valves close. When the ventricles contract, blood fills the atria again (venous - right and arterial - left).
3. The aortic and pulmonary valves open. This also occurs under pressure when the ventricles contract and blood is pushed into large vessels and follows either to the lungs (from the right ventricle) or to all organs and tissues (from the left ventricle).
4. During relaxation of the ventricles, the pulmonary and aortic valves close. At this time, the atrioventricular valves open and blood again enters the ventricles from the atria for the next release into the bloodstream.
Functions of the mitral snort
This leaflet heart valve is located in the left chamber between the ventricle and atrium. When open, it performs the function of being the entrance for blood flow into the ventricle. When the heart muscle is in the systolic phase, the valve blocks the backflow of blood.
The medical history of the field of cardiology indicates that due to its structure, the mitral snort (bivalve) is the first to be recognized on ultrasound. Due to its anatomy, it reflects the ultrasound signal well. Due to the fact that the anterior valve of the snort has good plasticity and mobility, medical specialists can examine in detail the structure of the valve apparatus.