Today, not only medications, physical procedures and surgery are used to treat many diseases. Innovative methods appear every year, and one of them is artificial liquids, analogues of natural human ones. This is exactly what we are talking about when we talk about “synovial fluid prosthesis”. Its introduction into the joint cavity not only relieves the symptoms of arthrosis, but also affects the root cause.
Prostheses based on hyaluronic acid are produced in disposable syringes for single use
How it works
A sterile solution of the prosthesis is injected into the joint capsule, where it becomes a substitute for natural synovial fluid. The product improves the physiological characteristics of joints affected by arthrosis:
- normalizes the elasticity and viscosity of natural lubricant;
- restores metabolism in tissues and hydrodynamics in joints;
- improves nutrition of tissues around cartilage and bones;
- reduces mechanical impact on the joint;
- supports adequate functioning of cartilage.
The prosthesis compensates for the lack of natural synovial fluid in the joint
How long does the effect last?
After completing a course of intra-articular injections, the effect lasts on average for several months - from 6 to 12. One course involves 3-5 injections at a certain interval. How long the therapeutic effect will last depends on the severity of the lesion and the molecular weight of the substance.
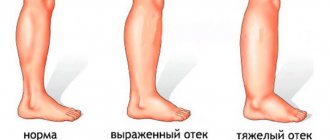
Synovial fluid prostheses bring maximum positive results in the initial stages of arthrosis. If degenerative-dystrophic changes have seriously progressed, it will not be possible to completely restore cartilage tissue. Replenishing the volume and viscosity of synovial fluid will alleviate symptoms and prevent complications.
Synovial fluid grafts help delay joint surgery
References
- National clinical guidelines for the diagnosis, treatment and prevention of venous thromboembolic complications. Association of Phlebologists of Russia, 2013-2017. — 51 p.
- Zubairov, D.M. Molecular basis of blood coagulation and thrombus formation, 2000. - 360 p.
- Practical recommendations for the prevention and treatment of thromboembolic complications in cancer patients, 2021. - 10 p.
- Egan, G., Ensom, M. Measuring anti-factor xa activity to monitor low-molecular-weight heparin in obesity: a critical review. Can J Hosp Pharm., 2015. - Vol. 68(3). — P. 33-47.
- Berges, A., Laporte, S., Epinat, M. et al. PROPHRE.75 Study Group. Anti-factor Xa activity of enoxaparin administered at prophylactic dosage to patients over 75 years old. Br J ClinPharmacol., 2007. - Vol. 64(4). — P. 428-38.
- Guyton, C., Hall, E. Textbook of Medical Physiology. 13th Edition, 2015. - P. 1075-1093
To whom are they shown?
Since 2003, WHO has officially recommended the use of hyaluronic acid-based drugs for the treatment of mild and moderate forms of osteoarthritis. Most often, this technique is used when conservative methods are ineffective.
The main indications for the use of prostheses are degenerative and post-traumatic diseases of the joints (arthrosis, osteoarthritis, arthrosis deformans), which are accompanied by painful discomfort and stiffness.
For the purpose of prevention, injections are prescribed in order to stop the progression of destructive processes in cartilage, when age-related changes in the joints lead to a deficiency of natural lubrication.
Synovial fluid prostheses are a real godsend for older people
Heparin gel I 1000IU/g 30g No. 1
Name
Heparin gel 1000IU/1g 30g
Description
The gel is colorless to light yellow with a specific odor.
Main active ingredient
Heparin sodium
Release form
Gel
Dosage
1000IU/1g
pharmachologic effect
Pharmacological studies show that when applied externally, Heparin gel has a pronounced antiexudative, antiedematous, antigranulomatous, anti-inflammatory and anticoagulant effect.
Indications for use
- symptomatic treatment and reduction of swelling and bruising after blunt trauma (for example, after a bruise); — symptomatic treatment of diseases of the superficial veins (varicose veins, phlebitis, periphlebitis, thrombophlebitis), varicose ulcers, varicophlebitis, conditions after removal of the great saphenous vein of the thigh as part of complex therapy.
Directions for use and doses
1-3 times a day, apply 3-10 cm of gel to the affected area of the skin and rub in gently. Due to limited experience with the drug in children and because there is insufficient research data, this drug should not be used in the treatment of children.
Use during pregnancy and lactation
Use during pregnancy is possible only if indicated under medical supervision. Can be used during lactation according to indications.
Impact on the ability to drive vehicles and other potentially dangerous mechanisms
The drug does not affect the ability to drive vehicles and potentially dangerous machinery.
Precautionary measures
If there are signs of bleeding, the possibility of using Heparin gel should be carefully considered. Heparin gel should not be used for bleeding, applied to open wound surfaces, weeping eczema or mucous membranes, as well as to infected areas in the presence of purulent processes. The penetration of heparin into healthy skin has been described in the case of topical application; Therefore, if thromboembolic complications are suspected, a platelet count should be checked to differentiate heparin-induced thrombocytopenia type II. Therefore, regular platelet count checks in patients with a history of thromboembolic complications are necessary whenever heparin is used (before starting heparin, on the first day after starting use, and then regularly every 3-4 days during treatment until the end of treatment). Heparin gel contains methyl parahydroxybenzoate and propyl parahydroxybenzoate as excipients, so it cannot be used in patients with allergies to parabens.
Use in pediatrics
Due to limited experience with the drug in children and because there is insufficient research data, the gel should not be used in the treatment of children.
Interaction with other drugs
The anticoagulant effect of heparin is enhanced by the simultaneous use of anticoagulants, antiplatelet agents and non-steroidal anti-inflammatory drugs. Ergot alkaloids, thyroxine, tetracycline, antihistamines and nicotine reduce the effect of heparin.
Contraindications
- known hypersensitivity to heparin or any component of the drug; - history of heparin-induced thrombocytopenia type II (immune-mediated HIT type II).
Compound
per tube: active substance - heparin (in the form of sodium heparin) - 1000 IU/g; excipients - carbopol 980 NF, levomenthol, methyl parahydroxybenzoate (E-218), propyl parahydroxybenzoate (E-216), rectified ethyl alcohol from food raw materials, triethanolamine, purified water.
Overdose
Due to low systemic absorption, overdose is unlikely. To date, the phenomena of overdose when using Heparin gel have not been described. With prolonged use on large surfaces, hemorrhagic complications are possible. Treatment: drug withdrawal. If necessary, use a heparin antagonist - protamine sulfate (1% solution).
Side effect
Allergic reactions to heparin after applying the drug to the skin are very rare. However, in some cases, hypersensitivity reactions may occur, such as skin redness and itching, which quickly disappear after stopping use of the drug. Incidence of heparin-induced antibody-mediated thrombocytopenia type II (platelet count
Storage conditions
Store at a temperature not exceeding 25°C. Keep out of the reach of children.
Buy Heparin gel d/nar.approx. 1000IU/g in tubes 30g in pack No. 1 in the pharmacy
Price for Heparin gel d/nar.approx. 1000IU/g in tubes 30g in pack No. 1
Instructions for use for Heparin gel d/nar. approx. 1000 IU/g in tubes 30 g in pack No. 1
Are there any contraindications
Injections of preparations based on hyaluronic acid are contraindicated if:
- there is local inflammation in the joint, because against the background of the inflammatory process, hyaluronic acid is destroyed and does not work;
- there is a history of autoimmune pathologies, since an organism with an impaired immune response may react negatively to such therapy;
- intolerance to synovial fluid of animal origin has been recorded (to prevent serious allergic reactions, including anaphylactic shock, it is necessary to undergo a tolerance test for the analogue);
- the patient is under 18 years of age.
During pregnancy and lactation, injections of hyaluronic fluid prostheses are contraindicated
Brief information about heparin
Heparin is produced by Russian, Ukrainian and Belarusian pharmacological enterprises in the form of a gel and injection solution.
The main active ingredient is sodium heparin with the addition of additional substances.
Being a direct anticoagulant, heparin, together with other drugs, can eliminate the following problems:
- blood clots in blood vessels;
- embolism;
- atrial fibrillation;
- unstable angina;
- thrombohemorrhagic syndrome;
- mitral heart disease;
- subcutaneous hematomas, etc.
Due to its ability to maintain a fluid state of blood, heparin is used for blood purification (hemodialysis), during artificial circulation and for laboratory tests.
The main contraindications to the use of heparin include:
- individual intolerance;
- increased bleeding;
- pathological changes in the liver and kidneys;
- leukemia;
- period of pregnancy and breastfeeding;
- intracranial injuries;
- ulcerative lesions of the gastrointestinal tract, etc.
Heparin is prescribed by the attending physician based on the symptoms of the disease and the absence of contraindications.
The use of Heparin can lead to the following problems:
- allergic reactions;
- thrombocytopenia;
- upset stomach, vomiting;
- bleeding;
- ulcers and hematomas on the skin surface when using a topical agent.
If side effects occur, the drug is ineffective, or side effects occur, the drug should be replaced with a drug with similar characteristics.
There are a sufficient number of medicines on the shelves of pharmacies that are similar in their effects to heparin.
What to prepare for: are there any complications?
Products based on hyaluronic acid sometimes cause unwanted reactions:
- itching and heat at the injection site;
- skin irritation;
- hives;
- swelling of soft supra-articular tissues;
- soreness in nearby muscles;
- numbness and tingling in the limb (during the treatment of gonarthrosis);
- local inflammatory reactions;
- rarely – anaphylactic shock.
Each body responds to therapy individually, so it is impossible to be 100% sure of the success of treatment. Injections should be carried out in a medical office, under the supervision of a specialist. The procedure is technically different from standard intramuscular injections and is performed under ultrasound guidance. It should be done by a rheumatologist or orthopedic traumatologist with the appropriate skills.
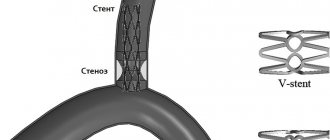
Prostheses are most often inserted into the knee, hip and shoulder joints
Detailed description of the study
The blood coagulation system (hemostasis) protects the body from blood loss. Typically, the clotting process - coagulation - is activated as a result of damage to blood vessels.
The coagulation system is based on the interaction of blood cells (platelets) and special proteins (clotting factors), which leads to the formation of a blood clot, stopping bleeding and restoring the integrity of blood vessels. Coagulation factors include: IIa (thrombin), XII, XI, X, Xa, etc.
Normally, over time, the blood clot is removed and the patency of the blood vessels is restored. As a result of some diseases, the formation of pathological blood clots - thrombi - occurs. Blood clots that separate (“break off”) from the vascular wall and spread with the bloodstream throughout the body are called emboli. The danger of emboli is that they can block the lumen of a vessel and completely stop blood flow in an organ or part of it. The following conditions and diseases can lead to thromboembolic complications:
- Thrombosis of the veins of the lower extremities;
- Oncological diseases;
- Postoperative period, especially after extensive surgical interventions;
- Severe chronic heart failure, myocardial infarction;
- Lung diseases with severe respiratory failure;
- Infectious toxic shock (sepsis);
- Obesity;
- Pregnancy and the first 6 weeks after birth;
- Prolonged bed rest, physical inactivity;
- Nephrotic syndrome, etc.
To prevent and treat thrombosis in clinical practice, various drugs of the antithrombotic group are used, in particular, anticoagulants - drugs that reduce blood clotting. These include heparins, which, in turn, are divided into unfractionated (UFH) and low-molecular-weight (LMWH). Both types of heparins prevent the formation and spread of blood clots, but UFH has more undesirable effects. Therefore, preference in clinical practice is given to LMWH.
The mechanism of action of both drugs is based on enhancing the anticoagulant activity of antithrombin III (plasma protein) and on the inactivation of coagulation factors IIa, Xa and some others. As a result, blood clotting is reduced. However, there is a risk of bleeding with minimal damage to the vessel wall, which has led to the need to monitor therapy in patients receiving heparin or LMWH.
The study of anti-Xa activity is aimed at determining the effectiveness of treatment with drugs from the heparin group (UFH and LMWH). This analysis is also relevant in patients who are taking other drugs that inhibit factor Xa - rivaroxaban, apixaban and other direct oral anticoagulants.
The test is based on the so-called. chromogenic analytics: in laboratory conditions, the interaction between the plasma sample under study and factor Xa is carried out. Based on the residual factor Xa detected using a chromogenic substrate, a graphical curve with a quantitative indicator of the drug is determined.
The result obtained must be compared with the type of anticoagulant taken and its dose. For patients taking low molecular weight heparins, this analysis is one of the basic methods for assessing the effectiveness of therapy. Usually the study is carried out 3-4 hours after administration of LMWH. For direct oral anticoagulants, indicators may vary - from 1 to 4 hours after using the drug.
Manufacturers of synovial fluid prostheses based on hyaluronic acid
| The product's name | Manufacturer country |
| Fermatron | Great Britain |
| Synvisc | USA |
| Hyalurome CS | Romania |
| Sinokorm | Austria |
| Visco Plus | Sweden |
| Go-on | Ireland |
| Ostenil | Germany |
| Giruan-Plus | Korea |
| RusVisk, Giastat | Russia |
Preparations based on hyaluronic acid have very different effects. How these drugs differ from each other, how effective drugs from different manufacturers are - the opinion of a specialist, supported by the arguments of evidence-based medicine:
Phlebotonics in the treatment of chronic venous insufficiency of the lower extremities
Currently, practicing doctors are witnessing the rapid development of the pharmaceutical market in our country. New drugs, both foreign and domestic, appear quite regularly. The arsenal of pharmacological agents used to treat a particular disease is constantly growing, which creates certain difficulties in choosing them. The situation is even more complicated by the fact that different manufacturing companies, as a rule, give their names to drugs based on the same active ingredient. In this abundance of information, it is of paramount importance to know and highlight the main links in the pathogenesis of the disease, on the basis of which principles and treatment tactics are developed.
Today, the misconception about the omnipotence of drugs is becoming more and more widespread. There are many reasons for this, and it would not be an exaggeration to name the main one as the active advertising of a particular pharmacological drug by the manufacturer. As a result, many patients, especially older ones, take a dozen different medications, periodically getting confused about the dosages and frequency of their use, and with each visit to the doctor they change this “set of pills” and at the same time suffer very significant financial losses.
The problem of inadequate prescription of drugs has not escaped the treatment of chronic venous insufficiency (CVI) of the lower extremities. If just a few decades ago the surgical method of treatment was the leading one and, moreover, was considered the only one worthy of serious attention, now another extreme is often observed - attempts to present pharmacotherapy as the basis of treatment, and consider other methods of treatment as an addition to the “pharmacotherapeutic basis”.
Considering the above, we consider it necessary to determine the tactics for using modern phlebotropic drugs in patients with CVI of the lower extremities. Phlebotropic drugs are basic in the treatment of CVI. Of course, severe disorders of the outflow from the lower extremities necessitate the use of drugs from other pharmacological groups - disaggregants, non-steroidal anti-inflammatory drugs, antibiotics, enzymes, etc. However, their prescription should be approached with caution, and the period of their use should be clearly limited to the implementation of a particular task treatment.
The main goals of drug treatment for CVI of the lower extremities are: relief of symptoms of CVI, prevention of complications, prevention in risk groups, preoperative preparation, postoperative rehabilitation.
Based on the pathogenesis of CVI of the lower extremities, the objectives of conservative therapy can be presented as follows: increasing the tone of the venous wall, improving lymphatic drainage, influencing microcirculatory changes, correcting hemorheological disorders, relieving inflammatory manifestations.
As mentioned above, phlebotropic drugs are the basis of drug treatment for patients with CVI. This is a fairly large group of pharmacological drugs that have the ability to increase venous outflow from the lower extremities. This effect of phlebotropic drugs is achieved by summing up the effects on various parts of the microvasculature, and in many of them, by a direct effect on the contractility of the venous wall. Of course, the phlebotonic effect should be recognized as the main effect of phleboprotective drugs. However, they all have a polyvalent mechanism of action: they stimulate lymphatic drainage, have anti-inflammatory activity, and improve hemorheology. It is this feature, which allows influencing the main links in the pathogenesis of CVI, that has united many drugs into the group of phleboprotectors and made them basic in the conservative treatment of patients with CVI of the lower extremities.
When should phlebotropic drugs be prescribed? The indication for the use of phleboprotective agents is the presence of symptoms of CVI of the lower extremities. It is necessary to immediately warn doctors against a very common mistake. The development of acute disturbance of blood outflow during thrombosis of the veins of the lower extremities does not require the use of this group of pharmacological agents. Moreover, the use of phlebotonics should be considered contraindicated in patients with acute venous thrombosis. During an acute thrombotic process, the venous wall is less susceptible to phlebotonic effects, and its implementation will only aggravate acutely occurring blood outflow disorders.
In the future, in the case of the development of post-thrombotic disease, when the processes of post-thrombotic occlusion and recanalization in various venous segments are balanced, when pathological venous refluxes of blood are formed and, therefore, one or another degree of CVI occurs, the indications for the prescription of phleboactive drugs should be considered justified.
An absolute indication for the use of phleboprotectors are the so-called functional (orthostatic and hormone-produced) phlebopathies. In these cases, lifestyle changes with the exclusion of the producing factor, taking phlebotonics and elastic compression have a clear positive effect.
And finally, the use of phleboprotective drugs is certainly indicated for varicose veins of the lower extremities - a disease that is the most common cause of the development of CVI.
In patients with early stages of varicose veins, when there are no symptoms of decompensation of blood outflow yet, and aesthetic complaints prevail, the main purpose of using phleboprotectors is mainly to prevent the progression of the disease. It is advisable to prescribe phlebotonics to such patients in short (1–1.5 months) courses. The interval between courses of pharmacotherapy should be 3–4 weeks.
The appearance of edema of the lower extremities is a reliable sign of decompensation of blood outflow. In patients with edematous syndrome, the objectives of pharmacotherapy are: relief of manifestations of CVI (edema, heaviness in the lower extremities), improvement of lymphatic drainage, correction of microcirculatory disorders, prevention of disease progression. For patients with decompensation of blood outflow, phlebotonics are prescribed in longer courses (2–2.5 months). The time interval between courses should not exceed 1 month.
In patients with trophic disorders of the soft tissues of the lower extremities, special care should be taken when choosing a conservative therapy program. The severity of trophic changes in the skin dictates the need to prescribe drugs belonging to various pharmacological groups, and the stages of the wound process and the tendency of patients to allergic reactions dictate a careful individual selection of drugs. Indications for the use of various groups of drugs depend on many factors, including the stage and severity of the disease, and the risk of complications. Drug treatment should be prescribed depending on the phase of the wound process.
At the first stage, when manifestations of acute inflammation and destruction of soft tissues predominate, the main goals of using pharmacological drugs are to quickly eliminate the symptoms of inflammation and fight infection. During this period, therapy is carried out with non-steroidal anti-inflammatory drugs (diclofenac, indomethacin, ketoprofen, meloxicam, etc.), antiplatelet agents (acetylsalicylic acid, dipyridamole, clopidogrel), antihistamines (ketotifen, clemastine, promethazine), antioxidants (vitamin E, emoxypine, mildronate) . For a long time it was believed that the absolute indication for the use of antibiotics was the presence of a trophic ulcer of the lower extremities. Currently, views on this problem have changed somewhat. Indications for antibacterial therapy: extensive trophic disorders occurring with severe perifocal inflammation, the presence of purulent discharge from a trophic ulcer. Local use of antibiotics is currently recognized as ineffective. Taking into account the microbial landscape, the most effective antibacterial agents are semisynthetic penicillins, cephalosporins of the second and third generations, and fluoroquinolones. We consider the use of phlebotropic drugs during this period inappropriate.
Thus, the main goals of pharmacotherapy for CVI of the lower extremities in the stage of trophic disorders at the first stage are to relieve inflammation in the area of trophic disorders, treat complications, and stop the process of tissue destruction. Basic agents: antiplatelet agents, antibiotics, antihypoxants, non-steroidal anti-inflammatory drugs, antihistamines.
At the second stage, when the process of tissue destruction is stopped and the phenomena of acute inflammation are stopped, the main task of pharmacotherapy is the correction of microcirculatory disorders. At this stage, it is necessary to create conditions for the “start” of tissue regeneration, the transition from the catabolic phase to the anabolic phase. During this period, polyvalent phlebotropic drugs are prescribed, and the use of antiplatelet agents and antioxidant therapy is continued.
So, the main goals of pharmacotherapy for CVI of the lower extremities in the stage of trophic disorders at the second stage are: initiation of tissue repair processes, reduction of symptoms of venous insufficiency. Basic means: phlebotropic drugs, antiplatelet agents, antioxidants.
Complete relief of inflammatory manifestations and the beginning of active epithelization of the ulcerative defect indicate the success of the treatment, and at this third stage the main goal is to consolidate the achieved effects, and the main remedy is phlebotropic drugs.
It should be noted that effective treatment of CVI of the lower extremities is possible only by using an integrated treatment approach using surgical methods, elastic compression and modern pharmacological agents. Therefore, it is advisable to use phlebotropic drugs both before surgery and as part of postoperative rehabilitation treatment.
Below we will dwell in more detail on some modern phleboprotective drugs, our own experience of using which allows us to recommend them for use in the treatment of patients with CVI of the lower extremities.
The composition of the drug Detralex includes 450 mg of diosmin and 50 mg of hesperidin. The main mechanisms of action of this drug for CVI are: increasing the tone of the veins, stopping inflammatory reactions, stimulating lymphatic drainage, eliminating microcirculatory disorders.
According to our data, the use of Detralex in more than 250 patients with initial forms of CVI in most cases (83%) causes significant clinical improvement, and also has a positive effect on the hemodynamics of the veins and the efficiency of the calf pump. It is also necessary to note the high safety of this phlebotropic drug. Even with long-term (12 months) use of Detralex, side effects occurred extremely rarely; treatment tolerability was assessed as moderate to high by 93% of patients.
When studying microcirculatory blood flow using laser Doppler flowmetry, it was possible to reveal that by the end of the course of conservative treatment in patients with varicose veins, there was a significant increase in the studied indicator, indicating increased perfusion of soft tissues (Fig.).
| Drawing. Dynamics of the microcirculation index in the main and control groups at different periods of treatment |
The drug Ginkor Fort contains 14 mg of gingko biloba extract, 300 mg of troxerutin and 300 mg of heptaminol hydrochloride. Gingko biloba extract has an antioxidant, antihypoxic effect, realized by suppressing the synthesis and release of inflammatory mediators and oxidation reactions involving free oxygen radicals. The antiaggregation and angioprotective effect of the drug is due to the suppression of the production of phosphodiesterase, which leads to the accumulation of cycloguanidine aminophosphoric acid in smooth cells and a decrease in arteriolar tone. Troxerutin belongs to the group of rutosides. Its angioprotective effect is realized by blocking hyaluronidase and thereby achieving stabilization of hyaluronic acid, one of the main components of cell membranes and interstitial substance. Heptaminol, in addition to affecting the main factor of venous return - the “muscular-venous pump”, increases the contractile activity of the right chambers of the heart, promotes adequate blood oxygenation and protects the vascular endothelium from hypoxia.
An important feature of the drug Ginkor Fort is the possibility of its use in the second and third trimesters of pregnancy. Studies have confirmed the effectiveness of this drug, which is used to relieve symptoms of CVI during pregnancy.
Recently, a new phlebotropic drug, Antistax, has appeared on the domestic market. The active ingredient of this drug is an extract of red grape leaves containing pharmacologically active bioflavonoids, the main of which are quercetin glucuronide and isoquercetin. The drug has a protective effect on the vascular epithelium, stabilizing the membranes, increases the elasticity of blood vessels, normalizing vascular permeability. Reducing the permeability of the vascular wall to plasma, proteins and water from the vessels into the surrounding tissue slows down the formation of edema and reduces existing edema.
The drug is available in the form of capsules containing 180 mg of active substance. Indications for use are initial forms of CVI, manifested by “heavy legs” syndrome, edema, and decreased tolerance to static loads. The drug is taken 1 capsule 2 times a day.
The results of several large clinical studies on the effectiveness of the drug Antistax, used in the treatment of patients with CVI, have been published in the foreign literature. Its positive effect on the relief of edematous syndrome (according to volumetric data) and other manifestations of CVI (pain, fatigue, heaviness in the legs) has been shown.
The initial experience of using Antistax in domestic clinical practice can be considered encouraging. It should be noted that this drug is well tolerated and has no significant side effects.
Thus, modern phlebotropic drugs, due to their polyvalent mechanism of action, high bioavailability, and mild side effects, are an effective means of pharmacological correction of CVI. Their use in combination with surgical treatment, elastic compression, sclerotherapy, and physiotherapy allows one to achieve good results in the treatment of patients with chronic impaired blood outflow from the lower extremities.
Yu. M. Stoyko , Doctor of Medical Sciences, Professor N. A. Ermakov , Candidate of Medical Sciences, National Medical Center named after. N. I. Pirogova, Moscow
Comparison of natural and artificial hyaluronic acid by molecular weight
| GK type | Molecular weight (MM) | Characteristics |
| Natural | 3 140 000 Yes | Constantly updated, normally has excellent shock-absorbing and lubrication properties |
| Artificial with low mm | 500,000 – 800,000 Yes | It is quickly removed from the joint, therefore it requires a large number of injections and frequent repetitions of courses |
| Artificial with medium mm | 800,000 – 2,500,000 Yes | The standard course includes 5 injections, lasts a relatively short time, and is used most often |
| Artificial with high mm | More than 2,500,000 Yes | Requires fewer injections per course and lasts longer |
Heparin
Heparin sodium is administered subcutaneously, intravenously, bolus or drip.
Heparin sodium is prescribed as a continuous intravenous infusion or as regular intravenous injections, and also subcutaneously (in the abdomen).
Heparin sodium should not be administered intramuscularly due to the risk of developing intramuscular hematomas.
The usual site for subcutaneous injections is the anterolateral abdominal wall (in exceptional cases in the upper arm or thigh), using a thin needle that should be inserted deeply, perpendicularly, into a fold of skin, held between the thumb and forefinger until the injection is complete. solution. The injection sites should be alternated each time (to avoid the formation of a hematoma). The first injection must be performed 1-2 hours before the operation; in the postoperative period - administered for 7-10 days, and if necessary - for a longer time.
The initial dose of sodium heparin administered for therapeutic purposes is usually 5000 IU and is administered intravenously, after which treatment is continued using subcutaneous injections or intravenous infusions.
Maintenance doses are determined depending on the method of administration:
- for continuous intravenous infusion, 1000-2000 IU/hour (24000-48000 IU/day) is prescribed, diluting sodium heparin with 0.9% sodium chloride solution;
- with regular intravenous injections, 5000-10000 IU of sodium heparin is prescribed every 4-6 hours;
— for subcutaneous administration, 15,000-20,000 IU is administered every 12 hours or 8,000-10,000 IU every 8 hours.
Laboratory monitoring of the effectiveness and safety of sodium heparin therapy
The dose of heparin sodium should be adjusted based on laboratory blood clotting parameters. When using heparin sodium, it is necessary to monitor the activated partial thromboplastin time (aPTT) or blood clotting time (BCT). The administered dose of sodium heparin is considered adequate if the aPTT is 1.5 to 2.0 times the normal values or if the patient's ICR is 2.5 to 3.0 times the control values.
With continuous intravenous infusion of heparin sodium, it is recommended to determine the initial aPTT, then determine the aPTT every 4 hours, followed by increasing or decreasing the rate of infusion of heparin sodium until the target aPTT level is achieved (1.5-2.0 times higher than normal), then determine the aPTT every 6 hours.
When bolus intravenous administration of heparin sodium is recommended, it is recommended to determine the initial aPTT, then determine the aPTT before each bolus, followed by an increase or decrease in the administered dose of heparin sodium.
When administering sodium heparin subcutaneously, it is recommended to monitor the aPTT 4-6 hours after injection with a subsequent increase or decrease in the administered dose of sodium heparin.
When using sodium heparin in low doses to prevent thromboembolic complications, it is not necessary to monitor the aPTT.
Continuous intravenous infusion is the most effective way of using sodium heparin, better than regular (periodic) injections, because provides more stable hypocoagulation and is less likely to cause bleeding.
Use of heparin sodium in special clinical situations
Primary percutaneous coronary angioplasty in acute coronary syndrome without ST-segment elevation and in myocardial infarction with ST-segment elevation: sodium heparin is administered intravenously as a bolus at a dose of 70-100 IU/kg (if the use of glycoprotein IIb/IIIa receptor inhibitors is not planned) or at a dose of 50 -60 IU/kg (when used together with inhibitors of glycoprotein IIb/IIIa receptors).
Thrombolytic therapy for myocardial infarction with ST segment elevation: sodium heparin is administered intravenously as a bolus at a dose of 60 IU/kg (maximum dose 4000 IU), followed by intravenous infusion at a dose of 12 IU/kg (not more than 1000 IU/h) for 24- 48 hours. The target APTT level is 50-70 seconds, which is 1.5-2.0 times higher than normal; APTT monitoring - 3, 6, 12 and 24 hours after the start of therapy.
Prevention of thromboembolic complications after surgery using low doses of sodium heparin: sodium heparin is injected subcutaneously, deep into the fold of the abdominal skin. The initial dose is 5000 IU 2 hours before surgery. In the postoperative period: 5000 IU every 8-12 hours for 7 days or until the patient’s mobility is completely restored (whichever comes first). When using sodium heparin in low doses to prevent thromboembolic complications, it is not necessary to monitor the aPTT.
When preventing thrombus formation in the postoperative period, the first injection should be performed 1-2 hours before the start of surgery; in the postoperative period, administer for 7-10 days, and if necessary, for a longer period.
Use in cardiovascular surgery during operations using the extracorporeal circulation system: the initial dose of sodium heparin is at least 150 IU/kg. Next, sodium heparin is administered by continuous intravenous infusion at a rate of 15-25 drops/min, 30,000 IU per 1 liter of infusion solution. The total dose is usually 300 IU/kg (if the expected duration of the operation is less than 60 minutes) or 400 IU/kg (if the expected duration of the operation is 60 minutes or more).
Use for hemodialysis: the initial dose of heparin sodium is 25-30 IU/kg (or 10,000 IU) intravenously as a bolus, then a continuous infusion of heparin sodium 20,000 IU/100 ml of 0.9% sodium chloride solution at a rate of 1500-2000 IU/h (if otherwise not stated in the instructions for use of hemodialysis systems).
Use of heparin sodium in pediatrics: Adequate controlled studies of the use of heparin sodium in children have not been conducted. The recommendations presented are based on clinical experience.
Initial dose: 75-100 IU/kg IV bolus over 10 minutes. Maintenance dose: children aged 1-3 months - 25-30 IU/kg/h (800 IU/kg/day), children aged 4-12 months - 25-30 IU/kg/h (700 IU/kg/day day), children over 1 year - 18-20 IU/kg/h (500 IU/kg/day) intravenously.
The dose of heparin sodium should be adjusted based on blood coagulation parameters (target aPTT 60-85 seconds).
Switching to warfaria therapy: To ensure sustained anticoagulant effect, full dose heparin sodium therapy should be continued until a stable target INR level (international normalized ratio) is achieved. After this, the administration of sodium heparin must be stopped.
Switching to dabigatran therapy: Continuous intravenous heparin sodium should be discontinued immediately after the first dose of dabigatran. With fractional intravenous administration, the patient should take the first dose of dabigatran orally 1-2 hours before the scheduled administration of the next dose of sodium heparin.
Is there an alternative
The human body contains the enzyme hyaluronidase, which breaks down hyaluronic acid even with high molecular weight. In addition, drugs can cause allergic and infectious reactions due to animal origin. Therefore, despite the innovative approach, they are not a perfect therapeutic agent.
It is safer and more effective to use the synovial fluid prosthesis “Noltrex”. In terms of molecular weight (more than 10,000,000 Da), it outperforms absolutely all products based on hyaluronic acid. Since specific enzymes do not act on the polymer, it remains in the body longer than other analogues, while also effectively restoring the properties of the joint fluid.
Noltrex reduces pain, improves the biomechanics of movements and stabilizes the joint for a long time - sometimes the effect lasts up to two years, which is impossible to achieve with hyaluronic acid.
The use of low molecular weight heparins in obstetric practice
D
Currently,
thrombosis and thromboembolic complications remain the leading cause of death in most developed countries
. In the United States alone, about 2 million people die annually from arterial and venous thrombosis, and approximately the same number of patients annually manage to survive episodes of deep venous thrombosis, thromboembolism, cerebrovascular thrombosis, transient ischemic attacks, coronary thrombosis, retinal thrombosis, etc. Even from malignant neoplasms, approximately four times fewer patients die. This indicates that thrombosis is an extraordinary cause of morbidity and mortality in the population, including maternal mortality. According to generalized data from the world literature, there are 2-5 thrombotic complications per 1000 births. 50% of all venous thromboembolic complications occur in women under the age of 40 years and, as a rule, they are associated with pregnancy.
Even with a physiologically proceeding pregnancy, especially in the third trimester, hypercoagulation occurs, which is primarily associated with an almost 200% increase in blood clotting factors I, II, VIII, IX, X in combination with a decrease in fibrinolytic activity and natural anticoagulant (antithrombin III and protein S) activity. In addition, in the third trimester, the speed of blood flow in the veins of the lower extremities decreases by half, which is partly due to mechanical obstruction of the venous outflow by the pregnant uterus, and partly due to a decrease in the tone of the venous wall due to hormonal changes in the body during pregnancy.
| There are 2-5 cases of thrombotic complications per 1000 births. |
Thus, the tendency toward blood stasis combined with hypercoagulability creates conditions that favor an increased risk of thrombosis.
Additional risk factors for thrombotic complications may include age (over 35 years), cardiovascular pathology, endocrine disorders, gestosis, kidney disease, purulent-septic diseases, as well as a number of acute conditions (placental abruption, amniotic fluid embolism, prolonged retention of a dead fetus in the uterus, etc.). Hypercoagulation is replaced by intravascular coagulation, manifested by various forms of disseminated intravascular coagulation (DIC).
It should be noted that expanding the indications for cesarean section is also associated with an increased risk of thrombosis due to surgery, significant changes in metabolism, trauma, entry of thromboplastic substances into the bloodstream, immobilization, slowing of venous blood flow, etc.
A special place among the risk factors for thromboembolic complications is occupied by purulent-septic processes in the pelvic area
, since the iliac, ovarian, and uterine veins are involved in the pathological process, which can be complicated by bacterial pulmonary embolism. At the same time, the increased concentration of highly dispersed plasma proteins (in particular, fibrinogen) additionally mediates increased structural hypercoagulation.
Over the past decade, the clinical picture has been enriched by the possibility of elucidating a number of previously unknown pathogenetic forms of thrombosis: immune, as well as genetic or so-called hereditary hemostasis defects that predispose to thrombosis.
To immune forms
include thrombosis caused by heparin-induced thrombocytopenia (HIT), thrombosis associated with the circulation of antiphospholipid antibodies in antiphospholipid syndrome, as well as a relatively recently discovered new form of immune thrombosis caused by autoantibodies to von Willebrand factor. In all immune thromboses, regardless of genesis, intravascular platelet aggregation occurs.
In recent years, the view on the pathogenesis of immune thrombosis has changed significantly. If previous concepts were reduced to the inhibition of pathophysiologically important natural antithrombotic agents (antigens) by antibodies, now the main role is given to the binding of antibodies through various proteins to blood cells (platelets, etc.) or the membrane of endothelial cells with subsequent activation of prothrombotic mechanisms by these cells through their FcgRII receptors or through the complementary cascade.
The mechanisms of heparin-induced thrombocytopenia and thrombosis caused by HIT are considered to be the most studied today.
There are
2 types of HIT
: the most common type I has an early onset, accompanied by mild thrombocytopenia, possibly associated with the ability of heparin fractions (mostly unfractionated), which do not have anticoagulant activity, to enhance small platelet activity; Type II causes sporadic, isolated cases of late-onset severe thrombocytopenia, which are immunoinduced and often associated with catastrophic thrombosis.
The treatment of thrombophilic conditions and DIC syndrome is based on eliminating the immediate cause
their occurrence (for example, antibiotic therapy for purulent-septic processes), as well as the impact on the main links of pathogenesis. A number of conditions in obstetrics dictate the need for preventive measures.
Indications for the prevention of thromboembolic complications during pregnancy and the postpartum period:
• pregnant women over 35–40 years of age
• pregnant women with extragenital pathology, especially with diseases of the cardiovascular system and kidneys
• multipregnant women with a burdened obstetric history (purulent-septic diseases, antenatal fetal death, fetal growth retardation, nephropathy, premature abruption of a normally located placenta)
• history of thrombosis and thromboembolism
• pregnant women who are indicated for surgery during pregnancy
• complicated course of pregnancy, childbirth and the postpartum period: (nephropathy, premature abruption of a normally located placenta, amniotic fluid embolism, purulent-septic diseases, massive blood transfusions)
• acute thrombosis and thromboembolism
• antiphospholipid syndrome
• genetic forms of thrombophilia.
The criteria for antithrombotic therapy in obstetric practice are its effectiveness and safety for the mother and fetus. Of the entire arsenal of antithrombotic agents (indirect and direct anticoagulants, antiplatelet agents, thrombolytics), sodium heparin and its derivatives have been and remain the drugs of choice.
. In obstetric practice, heparin sodium occupies a special place due to its immediate anticoagulant effect, the existence of an antidote, ease of dose management, and the absence of teratogenic and embryotoxic effects. Indirect anticoagulants pass through the placenta and have teratogenic and embryotoxic effects. In exceptional cases, their use is limited to the second trimester of pregnancy, when organogenesis is completed.
However, despite many advantages, conventional unfractionated or high molecular weight heparin has a number of undesirable side properties, which are mainly determined by its structure. Unfractionated heparin (UH) is a mixture of acidic macromolecular chains of sulfated mucopolysaccharide anions with a highly variable molecular weight from 4000 to 40,000 D.
As is known, the main effects of NG are antithrombin and antithromboplastin
. These effects are based on the interaction of the heparin-AT III complex with thrombin and the heparin-AT III complex with a number of coagulation factors (Xa, XIIa, XIa, IXa). To inhibit thrombin, at least 18 sugar residues in the heparin molecule are required, which is possible with a molecular weight of at least 5400 D. The ratio of anti-IIa and anti-Xa activity in NG is 1:1.
Due to the heterogeneity of its structure, NG has only 30% bioavailability, since it binds to many proteins and cells (macrophages, endothelial cells, etc.). In addition, NG is susceptible to the influence of antiheparin platelet factor (factor IV), forming a heparin-factor complex. This is fraught with the occurrence of heparin immune thrombocytopenia as a result of the formation of antibodies to this complex (the most dangerous form of thrombosis).
One of the undesirable effects of sodium heparin is the depletion of AT III with long-term use in large doses, which can also cause a hypercoagulable state and cause thrombosis. It is clear that increasing the dose of sodium heparin in such a situation does not lead to an anticoagulant effect.
When administered intravenously, the half-life of sodium heparin is 2 hours, which requires frequent administration of the drug; with subcutaneous administration, the half-life of NG increases due to prolonged absorption from the subcutaneous depot: in this case, it is possible to use NG 2 times a day after 12 hours. The therapeutic effect of NG is achieved by increasing the activated partial thromboplastin time (aPTT) by 1.5-2, 5 times compared to the norm. NG therapy requires regular laboratory monitoring due to the risk of hemorrhage, the main side effect of NG. Other side effects of NG include osteoporosis, alopecia, skin necrosis; a hypersensitivity reaction may occur.
low-molecular-weight heparins
have been actively introduced into clinical medicine , which have proven themselves to be excellent, since in most cases they exhibit greater antithrombotic activity and a significantly lower severity of hemorrhagic complications and other side effects.
LMWHs are obtained by depolymerization of NGs; their molecular weight ranges from 4 to 8 kDa. Depolymerization can be carried out by chemical, enzymatic and physical methods (g-radiation).
Changes in the structure of the heparin molecule, i.e. a decrease in molecular weight by almost 3 times entailed changes in pharmacodynamics and pharmacokinetics. LMWHs have higher bioavailability than NGs (about 98%) and a longer half-life. LMWHs bind less to various proteins and cells. Unlike NG, their renal clearance significantly prevails over cellular clearance (which is important to consider in patients with renal failure). In addition, LMWHs bind to endothelial cells to a much lesser extent than NGs, which also ensures long-term circulation in plasma (2-4 times longer).
NMG
do not have antithrombin properties and, therefore,
do not cause hypocoagulation
. The antithrombotic effect of LMWH mainly depends on its effect on factor Xa.
However, if the LMWH contains fractions with a molecular weight of more than 5400 D, which is equivalent to more than 18 disaccharide residues, then anti-IIa activity also appears. Thus, in one of the earliest LMWHs, nadroparin calcium, whose molecular weight is on average 4500 D, thanks to fractions with a molecular weight greater than 5400 D, the ratio of anti-IIa and anti-Xa activity is 1:4.
LMWH also promote the activation of fibrinolysis by releasing the tissue plasminogen activator t-PA from the endothelium; in addition, they are less susceptible to the action of antiheparin platelet factor IV and, accordingly, are less likely to cause heparin immune thrombocytopenia.
The antithrombotic effect of LMWH has long been associated exclusively with anti-Xa activity, until it became clear that only 30% of the activity of LMWH is carried out through AT III, and 70% through the so-called extrinsic coagulation pathway inhibitor TFPI, interaction with heparin cofactor II, inhibition of procoagulant actions of leukocytes, activation of fibrinolysis, modulation of the vascular endothelium (receptor- and non-receptor-mediated). This explains why patients remain in an “antithrombotic state” after subcutaneous administration of a prophylactic dose of LMWH for 24 hours, despite the fact that 12 hours after injection no anti-Xa activity is detected.
Progress in the field of hemostasiology has shown that activation of the extrinsic coagulation pathway and the release of tissue factor into the blood
(TF). This mechanism predominates during pregnancy, in the perinatal, postoperative periods, with purulent-septic diseases, antiphospholipid syndrome (APS), obesity, cancer and many cardiovascular diseases, as well as with a number of related conditions: heart defects, vena cava filter , percutaneous transluminal coronary angioplasty, pulmonary embolism, pulmonary distress syndrome, placental abruption, amniotic fluid embolism, etc.
TFPI factor, or lipoprotein-associated coagulation inhibitor (LACI factor), is a powerful natural inhibitor of the extrinsic coagulation pathway. LMWHs can significantly increase its level in the blood. The TFPI factor controls the factor Xa negative feedback mechanism and inhibits a number of complexes that, through the formation of prothrombinase, lead to the generation of thrombin and then fibrin.
TFPI has other pharmacological properties as a potential antithrombotic agent: it is an inhibitor of protease formation, a direct inhibitor of factor Xa and elastase, an inhibitor of TF-mediated activation of platelets and macrophages; it interacts with low-density lipoproteins with a change in their pathogenetic role (especially in atherosclerosis), interacts with the vascular endothelium, provides modulation of endogenous glycosaminoglycans, and neutralizes endogenously formed TF.
Under normal physiological conditions, TFPI is synthesized in the microvascular endothelium and in small amounts by megakaryocytes and macrophages and is not synthesized by normal hepatocytes or the endothelium of large vessels. Minor amounts of TFPI come from fibroblasts, but when these cells are activated, TFPI levels increase 6-8 times.
Returning to the effects of LMWH, it should be noted that regardless of the pathogenetic mechanism of thrombosis, what they have in common is the activation of the thrombin pathway, and the advantage of LMWH is their ability to prevent the formation of thrombin
. If we take into account the lesser dependence of the antithrombotic effect of LMWH on the level of AT III than that of NG, then we can think about the use of LMWH in patients with AT III deficiency.
Unlike NG, due to their lower molecular weight and greater bioavailability, LMWH circulate in the blood longer and provide a long-lasting antithrombotic effect in significantly lower daily doses. A single subcutaneous administration of the drug per day is possible: the drugs do not cause the formation of hematomas in the injection area.
LMWHs do not cause hypocoagulation, since the antithrombotic effect is aimed at inhibiting factor Xa and the extrinsic coagulation pathway; are much less susceptible to the influence of antiheparin platelet factor 4; therefore, they extremely rarely cause thrombocytopenia and do not cause immune thrombosis (Table 1).
Considering the mechanism of action of LMWH and the results of their use in widespread clinical practice, most researchers believe that there is no need for laboratory monitoring when using LMWH for prophylactic purposes. However, their anticoagulant effect can be assessed by anti-Xa activity. Biological methods for monitoring therapy with NG and LMWH, taking into account their effect on various components of the hemostatic system, are presented in Table 2.
Before the advent of LMWH, therapy control was aimed at ensuring an adequate dose of NG to avoid dangerous hemorrhagic complications. When using LMWH there is practically no problem of hypocoagulative effects. However, monitoring the effectiveness of the drug is very important. For this purpose, thrombophilia markers such as the thrombin-antithrommin complex, F1+2 prothrombin fragments, and especially fibrin-fibrinogen degradation products can be used. Markers of intravascular coagulation and thrombophilia are presented in Table 3.
The establishment of the absence of transplacental transfer of LMWH has opened up great opportunities for its widespread use in obstetric practice, especially in pregnant women with diseases of the cardiovascular system, with APS and in a number of conditions accompanied by thrombophilia and intravascular coagulation. The predominant effect of LMWH on the extrinsic blood coagulation pathway opens up an attractive prospect for the treatment of endothelial changes in preeclampsia.
Experience with the use of LMWH nadroparin calcium (Fraxiparin)
in obstetric practice indicates that LMWHs are the drugs of choice for the prevention of thromboembolic complications in pregnant women with artificial heart valves, since these patients require long-term (throughout pregnancy, childbirth) use of anticoagulants, as well as in pregnant women with a vena cava filter, in patients with a history of thrombosis and a deficiency of natural anticoagulants - AT III and protein C as a prevention of thromboembolic complications after cesarean section and in the postpartum period in high-risk groups for these complications. LMWHs have a positive effect in women with recurrent miscarriage and APS. Pathogenetically, this is justified due to the fact that LMWHs affect those hemostasis disorders that are induced by lupus anticoagulant, anticardiolipins, and their complexes, namely, disruption of the activation pathway and action of protein C, endothelial damage and disruption of the release of AT III, TFPI, prostacyclin and etc. Thus, LMWHs prevent the development of micro- and macrothrombosis in APS.
A positive property of LMWH (in particular, nadroparin calcium) is the relief of DIC syndrome in pregnant women with gestosis within 2-3 days
. As a rule, this is accompanied by regression of the disease. However, if the main manifestations of gestosis do not disappear, then LMWH therapy for more than 1 week is not advisable. Perhaps the observed positive effect of LMWH in pregnant women with initial forms of gestosis is due to its effect on the endothelium. In addition to the stabilizing effect of antiplatelet agents and anticoagulants, LMWHs prevent the expression of von Willebrand factor on the endothelium.
There are preventive and therapeutic doses of LMWH
. An important question remains about the duration of therapy, which depends on the underlying disease. Thus, in pregnant women with hereditary thrombophilias, it is necessary to use LMWH throughout pregnancy. Considering that in hereditary thrombophilias, as well as in a number of other cases, anticoagulant therapy is necessary throughout pregnancy, LMWH is the drug of choice also because it does not cause osteopenia with long-term therapy. In pregnant women with a vena cava filter, LMWH is used in the third trimester, during childbirth and in the postpartum period; with concomitant APS - throughout pregnancy with alternating prophylactic and therapeutic doses; in pregnant women with artificial heart valves, LMWHs are used from the third trimester of pregnancy.
Prevention of thromboembolic complications after cesarean section is especially relevant when several risk factors are combined: extragenital diseases (in particular, cardiac pathology), obesity, APS, etc. Its duration is at least 10 days. The prophylactic dose of one of the first and most studied LMWHs, nadroparin calcium (Fraxiparine), is usually 150 ICU/kg once daily subcutaneously (usually 0.3 mg). It should be noted that the anti-Xa activity of nadroparin calcium is most often measured in anti-Xa ICU units. 1 ICU corresponds to 0.41 international units of anti-Xa.
Fraxiparine solution is available in disposable syringes of 0.3, 0.4, 0.6, 1 ml. It is convenient to use, the injections are painless and do not leave bruises. The drug is administered under the skin of the abdominal wall, which makes it possible to use it on an outpatient basis.
Thus, the use of LMWH in obstetric practice opens up new prospects for the effective prevention and treatment of thromboembolic complications, diseases occurring with DIC syndrome, as well as shock and shock-like conditions.
Nadroparin calcium –
Fraxiparine (trade name)
(Sanofi-Synthelabo)