Norilsk Interdistrict Children's Hospital
A.I. Lobanov, O.G. Lobanova
The article discusses the problems of hemorrhagic disease of newborns with late onset. Currently, in maternity hospitals, the prevention of hemorrhagic disease is not carried out for all newborns. A clinical analysis of 9 cases of the disease was carried out. A dangerous feature of the late manifestation of hemorrhagic disease (after a month of life) is the development of massive intracranial hemorrhages against the background of increased bleeding. The late onset of hemorrhage due to vitamin K deficiency is characterized by a combination of three factors: lack of prevention of hemorrhagic disease, breastfeeding and transient cholestasis.
In the practice of a pediatric resuscitator, non-traumatic intracranial hemorrhages in children in the first months of life are quite rare. It is not always possible to determine the true cause of hemorrhage. At the same time, it is known that one of the reasons for the development of intracranial hemorrhages in children in the first months of life is late hemorrhagic disease of the newborn (LDH). It is impossible to predict the development of late onset of the disease, so prevention of tension-type headache at the maternity hospital stage is extremely important.
The development of HDN in newborns, especially those who are breastfed, is preceded by vitamin K deficiency: gamma-carboxylation of vitamin K-dependent blood coagulation factors is impaired in the hepatocyte. As a result, factors that have not undergone carboxylation lose their ability to participate in the blood clotting process. Immunologically, they are found in the blood in normal quantities, in the form of non-carboxylated and non-functioning molecules, denoted in the literature by the abbreviation PIVKA (Protein-Induced by Vitamin K Absence) [1].
These defective coagulation factors are not able to qualitatively influence blood coagulation processes, which leads to the development of tension-type headache. The most dangerous manifestation of this pathology is intracranial hemorrhage, which occurs against the background of general bleeding (skin hemorrhages, gastrointestinal bleeding).
For the period from 2004 to 2008. 9 children with non-traumatic intracranial hemorrhages were hospitalized in the intensive care unit of City Clinical Hospital No. 4 in Izhevsk. The age of the patients ranged from 1 to 2.5 months of life. Six children were hospitalized from home and three from city hospitals.
Anamnesis
When collecting anamnesis, it was found that all children were from an almost normal pregnancy, term birth, and were breastfed. In the maternity hospital, all newborns were vaccinated against hepatitis B and BCG. Prophylactic administration of sodium menadione (Vikasol) to newborns has not been carried out. On days 5-7, all children were discharged home. Subsequently, 2 children were vaccinated against hepatitis B twice.
Debut Clinic
The onset of the disease was almost the same in all children. 1-2 days before the occurrence of intracranial hemorrhage, hemorrhagic elements appeared on the skin or oral mucosa (Table 1).
Single deep ecchymoses with a diameter of 5 to 20 mm were found by parents more often on the extremities, less often on the torso. Small multiple hemorrhages on the oral mucosa were detected already during examination in the intensive care unit. One sick child had blood in the stool and prolonged bleeding from the injection site during repeated vaccination against hepatitis B. Upon consultation with a surgeon, no pathology was identified.
In all patients, intracranial hemorrhage manifested itself with sudden, painful, but short-lived crying. 8 children immediately developed constant and persistent vomiting, in two cases with blood. Vomiting was not observed in only one child. At first, the children showed periodic restlessness, began to moan, refused to feed, then became apathetic and indifferent to their surroundings. 7 children had tonic convulsions. The skin became increasingly pale. Short-term low-grade fever gave way to hypothermia.
Almost all children were hospitalized very late: the time spent at home from the moment of hemorrhage to hospitalization ranged from one day or more. Upon admission to the intensive care unit, 8 children were in extremely serious condition. All of them had decompensation of pulmonary ventilation, systemic circulatory disorders, focal and cerebral neurological symptoms, and disorders of coagulation hemostasis. In 6 cases, those admitted had rare, arrhythmic breathing or its complete absence. In 2 cases, respiratory disturbances were only in the form of severe tachypnea (respiratory rate - within 100/min).
All patients had severe pallor of the skin and mucous membranes with a cyanotic tint, as well as bleeding from the injection sites. In 2 children there were signs of mild pulmonary and gastric bleeding, in the form of hemorrhagic discharge from the endotracheal tube and gastric tube. Eight children had a slight jaundiced skin tone upon admission. The deficit in circulating blood volume ranged from 30 to 39% of the norm (the norm of bcc in children in the first months of life is 85 ml/kg; bccfact = body weight/weight part of bcc according to hematocrit in the table). Blood pressure ranged from 80/40 to 110/72 mmHg. Art. In 7 cases, children had tachycardia with dull heart sounds. In the neurological status of 6 children, disturbances in the central regulation of breathing were observed. Pathological forms of breathing in the form of severe bradypnea or apnea in these children developed even before hospitalization and continued to progress until the complete loss of automatic breathing. Brainstem reflexes from mucous membranes (cornea, pharynx, trachea) were not evoked. There was severe dryness and swelling of the oral mucosa and sclera. The oculocephalic reflex was absent. Fixed, bilateral, paralytic mydriasis and diffuse muscle atony were detected.
In all children, the large fontanel was bulging, dense to the touch, with no pulsation. Severe hypothermia of the scalp was noted. 5 children experienced attacks of hormetonia (periodic tonic tension of the muscles of the limbs and trunk, occurring against the background of muscle atonia spontaneously or under the influence of irritants, lasting no more than 10 s). In this case, the appearance of hormetonia is associated with damage to the trunk at the level of the midbrain and pons due to transtentorial herniation, when functional separation of the trunk and cerebral hemispheres occurs. As the coma deepened, the attacks of hormetonia stopped. Thus, all 6 patients were diagnosed with a 3rd degree coma (extraordinary). All patients admitted to the intensive care unit in a state of extreme coma died.
Two children were diagnosed with grade 1-2 coma upon admission to the intensive care unit. Tonic convulsions, transient symptoms of damage to the III, VI, VII pairs of cranial nerves, and tachypnea were noted. The large fontanelle was dense, bulging, but retained its pulsation. The reaction of the pupils to light, brainstem reflexes from the mucous membranes (cornea, pharynx, trachea), and oculocephalic reflex remained intact. One patient had signs of moderate intracranial hypertension in his neurological status.
Laboratory examination
In peripheral blood tests, most patients showed a significant decrease in hemoglobin levels (Table 2).
In all patients, blood clotting time (Morawitz) was sharply prolonged. The prothrombin index was determined in only one patient and was reduced. The platelet count was normal or elevated and tended to increase further as the day progressed. Normal fibrinogen levels were observed in 4 patients; in the rest, fibrinogen was not determined. Direct bilirubin remained elevated in 8 children, liver enzymes were slightly elevated in 1. Diagnosis
All children had subarachnoid hemorrhages. In 8 cases the hemorrhages were massive. Diagnosis of intracranial hemorrhages was carried out on the basis of anamnesis, clinical data and ultrasound examinations. Carrying out neuroimaging research methods (CT, MRI of the brain) in the vast majority of patients was impossible due to the extremely serious condition of the patients. In 3 patients, after the bleeding subsided, when intracranial hypertension (irreversible cerebral edema) was not extremely pronounced, the hemorrhage was confirmed by lumbar puncture (one day later). Liquor often flowed out under low or normal pressure and was red-brown in color. When centrifuged, sharp xanthochromia was revealed; microscopy revealed large numbers of altered (hemolyzed) red blood cells. A biochemical study of the cerebrospinal fluid determined low levels of glucose and high levels of protein and lactic acid (lactate). Therapy
8 children were on artificial ventilation. All patients underwent correction of blood volume, hemostatic and metabolic disorders, as well as anticonvulsant and neuroprotective therapy. Relief of bleeding began with the introduction of 10 mg of Vikasol through a tube, a single transfusion of fresh frozen plasma in a volume of 15 ml/kg. With the cessation of bleeding (usually after 10-12 hours), further administration of sodium menadione (intramuscular) was continued to create a depot of 5 mg/day for 2-3 days. Eight patients received red blood cell transfusion due to the development of posthemorrhagic anemia. Subsequently, none of the patients had any bleeding. Autopsy results
At autopsy, massive subarachnoid hemorrhages were discovered in the dead children; in 2 cases with the presence of blood also in the subdural space and in 1 case - in the ventricular system of the brain. In 2 cases the brain was in a state of necrosis. In addition, all the dead children had changes in the liver, most often in the form of nonspecific reactive hepatitis with extracellular cholestasis and initial fibrosis of the portal tracts.
Discussion
During clinical observation, it was noted that all children admitted to the intensive care unit with generalized bleeding were united by a number of common factors and clinical features characteristic of the late onset of HDN. The main factor is the lack of prevention of tension-type headache. The incidence of late onset tension-type headache without vitamin K prophylaxis, according to some data, is 5-20 cases per 100 thousand newborns [2]. None of the 9 children admitted to the intensive care unit with generalized bleeding were treated in the maternity hospital with the administration of sodium menadione.
The second factor is breastfeeding. All observed children were full-term and were exclusively breastfed. Under physiological conditions, vitamin K (K± or phylloquinone) enters the child’s body with food (breast milk) and is additionally synthesized in the intestine in the form of vitamin K2 or menaquinone. But the synthesis of vitamin K2 in the intestines occurs mainly by Bacteroides fragilis and some Escherichia coli - flora that is colonized during artificial feeding.
During natural feeding, the intestines are populated by Bifidobacterium, Lactobacillus and Clostridium - flora that are practically incapable of synthesizing vitamin K2 [3]. The third factor is the phenomenon of transient cholestasis. They were noted in 8 cases. Natural vitamin K is absorbed in the small intestine in the presence of bile and fat. A decrease in bile flow leads to malabsorption of fats and fat-soluble vitamins, increasing even greater vitamin K deficiency. Newborns, due to the immaturity of the excretory function of the liver, are especially prone to cholestasis [4].
In 8 out of 9 children admitted to intensive care after the age of 1 month of life, the level of direct bilirubin still remained elevated (from 12 to 27 mmol/l), which indicates transient, functional and excretory liver failure. Of the clinical features characteristic of the late onset of HDN, it should be noted: firstly, the development of massive intracranial hemorrhages in combination with skin hemorrhages and bleeding from the gastrointestinal tract. Secondly, the observed children did not have severe pathology at the onset of the disease with a high risk of developing DIC syndrome.
Almost on the eve of the development of late TTH, all children were examined by local pediatricians. Thirdly, late hospitalization of these children in the intensive care unit. The reason for the late hospitalization of the observed children was the fact that the increase in intracranial pressure in children in the first months of life occurs more slowly due to the fact that the child’s skull (before the fusion of the cranial sutures and the closure of the fontanelles) remains temporarily pliable and adapts to the emerging conditions. When blood enters the intrathecal space of the brain, the threatened symptoms are delayed for some time, so the parents sought medical help too late.
Fourthly, changes in peripheral blood and cerebrospinal fluid. These changes are adaptive reactions of a sanogenetic nature. They are caused by blood poured into the intrathecal space and its breakdown products. Therefore, the observed children experienced a reactive increase in body temperature, changes in peripheral blood were noted in the form of leukocytosis and a shift in the leukocyte formula to the left. The entry of the blood itself and its breakdown products into the cerebrospinal fluid space caused reactively expressed pleocytosis and hyperproteinrachia. These changes are also protective (sanogenetic) in nature and disappear as the cerebrospinal fluid is cleared of blood by the end of 2-3 weeks.
Blood breakdown products have pronounced toxicity (oxyhemoglobin, serotonin, bilirubin, etc.) and additionally cause severe cerebral ischemia, leading to cerebral infarction. And finally, another feature of late HDN is the hypocoagulation direction of the results of available tests for assessing hemostasis and their stabilization during the administration of vitamin K (Vikasol).
The basis of laboratory diagnosis of tension-type headache is the determination of prothrombin time and index, which reflect the total level of (three out of four) vitamin K-dependent coagulation factors (II, VII, X) (Table 3).
In TTH, the platelet count and thrombin time should be normal [5]. In 8 observed children, the prothrombin index was not determined, and in 1 it was reduced. None of the observations revealed a decrease in the number of platelets; on the contrary, in response to prolonged bleeding, compensatory thrombocytosis developed in 7 cases. This circumstance excludes the involvement of platelets in pathological consumption reactions associated with DIC syndrome.
In the absence of clinical signs of DIC, fibrinogen was not detected upon admission in more than half of the patients. This is due to a number of reasons, such as a decrease in the protein-synthetic function of the liver during the development of a critical condition, the influence of hypovolemia, hypoxia, and acidosis. In the classic form of HDN, which occurs in newborns in the maternity hospital, in contrast to its later form, intracranial hemorrhages, such severity and decompensation of vital functions do not occur.
Conclusion
Based on the clinical observation, it can be concluded that full-term newborns are susceptible to the occurrence of late tension-type headache, in whom a combination of factors such as the lack of prophylactic administration of sodium menadione, breastfeeding and transient cholestasis became possible. In newborns who are expected to breastfeed, prevention of tension-type headache is especially important.
REFERENCES 1. Barkagan 3. S. Introduction to clinical hemostasiology. - M.: Newdiamed-AO, 1998. - P. 21-23. 2. Dolgov V.V., Svirin P.V. Laboratory diagnosis of hemostasis disorders. - M.: Triada, 2005. - 213 p. 3. Gusel V. A., Markova I. V. Pediatrician’s Handbook of Clinical Pharmacology. - Leningrad, 1989. - pp. 161-163. 4. Shabalov N. P. Neonatology. Volume 2. - St. Petersburg, 1996. - P. 95. 5. Barkagan 3. S. Hemorrhagic diseases and syndromes. - M.: Medicine, 1988. - 498 p.
Read the article on the website www.evrika.ru
Late hemorrhagic disease of the newborn (clinical analysis)
Untimely nosological verification of hemorrhagic syndrome is often due to underestimation of anamnestic data, incorrect interpretation of clinical manifestations, as well as errors in the interpretation of laboratory examination results [1–4]. In addition, in some cases, the reason for a late diagnosis is terminological confusion, as a result of which a practicing pediatrician may have an erroneous idea about the age limits for certain types of hemorrhagic disorders. Thus, the generally accepted term “hemorrhagic disease of the fetus and newborn” (code P53 according to ICD-10) from a formal point of view should be used only in cases where vitamin K-deficiency coagulopathy is detected in the perinatal period. At the same time, it has been proven that vitamin K deficiency in a child’s body can clinically manifest itself not only during the newborn period, but also in subsequent weeks and even months of life [5–9]. In these cases they speak of “late hemorrhagic disease of the newborn.” However, the correctness of this term raises reasonable doubts, since it is used to refer to hemorrhagic syndrome that manifests itself in the postneonatal period. The erroneous association of vitamin K deficiency only with the neonatal period creates the prerequisites for ignoring these conditions when searching for the causes of hemorrhagic disorders in children over 1 month of age. Underestimation of the role of vitamin K deficiency in the genesis of hemorrhagic syndrome in the postneonatal period may cause late diagnosis and the risk of developing serious complications. As an example of late verification of vitamin K-dependent coagulopathy, we present our own clinical observation. Girl aged 1 month. 11 days with a diagnosis of “Gastroduodenal reflux? Esophagitis? was referred by a local pediatrician for hospitalization due to frequent regurgitation and the appearance of brownish streaks in the vomit. From the anamnesis we know: a girl from a somatically healthy woman, 26 years old. From the first pregnancy, which occurred with toxicosis in the first trimester. 1st birth, spontaneous at 38 weeks, physiological. Body weight at birth – 3100 g, height – 51 cm. Apgar score – 8–9 points. Attached to the breast immediately after birth. She took the breast actively. From the 3rd day of life, icterus of the skin was noted, which was regarded as a manifestation of physiological jaundice. In the maternity hospital, she was vaccinated against tuberculosis and hepatitis B. She was discharged from the maternity hospital on the 4th day in satisfactory condition. The girl has been breastfed freely since birth. Body weight gain during the first month of life was 1100 g. While breastfeeding, from the first days of life, slight regurgitation after feeding was noted, which was regarded as a manifestation of infant regurgitation; no therapy was carried out. Within 3 weeks. The child retained yellowness of the skin with gradual fading. In 1 week Before hospitalization, regurgitation became more frequent. Upon admission to the department, the child’s condition was of moderate severity. Moderate lethargy. The skin is pale. On the left shoulder and in the left breast area there are dense subcutaneous nodules up to 1.0 and 0.5 cm in diameter, respectively (according to the mother - “due to the clip-on closures on the vest”). Mucous membranes are moist and clean. Heart sounds are sonorous, rhythmic, gentle systolic murmur at the apex. Breathing in the lungs is puerile. The abdomen is soft and accessible to deep palpation. Liver +1.0 cm. Stool is independent, mushy, with a small admixture of mucus. Urination is free. There are no focal or meningeal symptoms. Reflux contains scanty streaks of blood. Upon admission, clinical and biochemical blood tests, a general urinalysis, and ultrasound of internal organs were examined with an additional assessment of the functional state of the gastroesophageal area (water-siphon test). In this case, mild normochromic, normocytic anemia was detected: HGB 106 g/l, RBC 3.4x1012, MCV 81 fl, MCH 26.1 pg, CP 0.93, moderate thrombocytosis: 612x109. A biochemical blood test revealed slight hyperbilirubinemia (total bilirubin - 30 µmol/l, direct bilirubin - 7 µmol/l) and a moderate increase in LDH (1020 units/l). Ultrasound data of internal organs indicated the absence of gastroesophageal reflux, chalasia and pyloric stenosis. During the 1st day of the child's stay in the department, attention was paid to continued bleeding from the sites where blood was collected for research. Taking into account the persistent hemorrhagic syndrome (bleeding from injection sites, streaks of blood in the vomit), the child underwent (cito!) a clinical blood test with reticulocytes, neurosonography and a coagulogram. At the same time, the anamnesis and clinical data were analyzed in detail, which made it possible to further identify the following: – the absence of hemorrhagic diseases in the family; – during pregnancy and after childbirth (this time period was studied, taking into account the feeding of the child exclusively with breast milk), the mother did not receive medications that could affect hemostasis; – menadione sodium bisulfite was not administered to the child in the maternity hospital; – streaks of blood in the refluxate appeared within 1 week. before hospitalization; – clearly limited change in skin color (like “bruises”) up to 0.5 and 1.0 cm in diameter above the “nodules” in the area of the nipple of the mammary gland on the left and left shoulder. Palpation of the indicated tumor-like formation in the area of the left mammary gland revealed hemorrhagic discharge from the nipple. After the outflow of hemorrhagic discharge, the “nodule” ceased to be palpable, but limited blueness of the skin in this place remained. All this made it possible to consider the identified “nodules” as subcutaneous hematomas, which the mother interpreted as a manifestation of skin traumatized by the clips on children’s clothing. Taking into account the ongoing bleeding and the emerging clinical signs of anemia, immediately after blood sampling, emergency syndromic therapy was started: intravenous administration of menadione sodium bisulfite (1 mg/kg), fresh frozen plasma (FFP) (20 ml/kg), a pressure bandage was applied to the area injections with a hemostatic sponge. Analysis of the results of the (cito!) examination revealed changes in the clinical blood test in the form of the appearance of hypochromic hyperregenerative anemia of moderate severity (Hb - 88 g/l, red blood cells - 3.2x1012, color index - 0.83, reticulocytes - 5.3% ), persistent thrombocytosis (621x109) and a normal level of bleeding duration (according to Duque - 2 minutes). At the same time, according to the coagulogram data, attention was drawn to the absence of coagulation along the internal (activated partial thromboplastin time (APTT) - no clot) and external (prothrombin index - no clot) coagulation pathways with normal fibrinogen levels (3.81 g/l) and final coagulation stage (thrombin time (TV) – 15.1 s) (Table 1). Neurosonography data allowed us to exclude intracranial hemorrhagic changes. The results of the laboratory examination made it possible to diagnose a violation of plasma hemostasis as a vitamin K-dependent coagulopathy. This was supported by: – absence of disturbances in primary hemostasis (neither thrombocytopenia nor prolongation of bleeding according to Duque was detected); – the presence of disturbances only in those links of secondary hemostasis, the functional activity of which is determined by vitamin K. Thus, there was pronounced hypocoagulation along the internal and external coagulation pathways, while fibrinogen and TB remained within normal limits. Thus, the anamnesis data, clinical picture and examination results made it possible to verify late hemorrhagic disease of the newborn, which determined the need to continue therapy with menadione with sodium bisulfite for another 3 days. It should be emphasized that the hemorrhagic syndrome in the child was relieved after the administration of FFP, while complete normalization of coagulogram parameters occurred only after completion of the course of treatment with menadione sodium bisulfite (Table 1). Taking into account the fact that vitamin K deficiency in the child’s body could be caused not only by a nutritional factor (exclusive breastfeeding, characterized by a low vitamin K content), but also by its insufficient synthesis by endogenous intestinal microflora, as well as by a violation of its utilization in the intestine, studies were carried out additional examinations. There were no clinical or laboratory signs of cholestasis or malabsorption. This made it possible to consider the lack of prophylactic administration of menadione sodium bisulfite in the early neonatal period and exclusive breastfeeding as the main provoking factors for the development of vitamin K-dependent coagulopathy in this patient. Final diagnosis: main – “Late hemorrhagic disease of the newborn”; complications – “Posthemorrhagic anemia of moderate severity”; accompanying – “Infantile regurgitation”. On an outpatient basis, it is recommended to continue breastfeeding if the mother follows a rational diet for nursing women and takes daily multivitamins. To correct anemia, a polymaltose complex of ferrous iron (5 mg/kg/day for elemental iron) was prescribed for 1 month. with subsequent monitoring of clinical blood test parameters.
The presented clinical case demonstrates the need for a pediatrician to be alert to any, even the most minimal, hemorrhagic manifestations, especially in young children. At the same time, replacing the widely used term “late hemorrhagic disease of the newborn” with “vitamin K-dependent coagulopathy” will avoid the erroneous association of vitamin K deficiency conditions exclusively with the neonatal period. Inclusion of this pathological condition in the list of possible causes of hemorrhagic syndrome in children, regardless of their age, will allow timely verification of the diagnosis and prescribing adequate therapy.
Literature 1. Barkagan L.Z. Hemostasis disorders in children. M., 1993. 2. Nelson Textbook of Pediatrics, 19th Edition /RM Kliegman, BM Stanton, J. St. Geme, N. Schor, R. E. Behrman. New York, London: Elsevier Inc., 2014. 3. Childhood diseases / ed. N.P. Shabalova / 6th ed. St. Petersburg: Peter, 2009. 4. Dolgov V.V., Svirin P.V. Laboratory diagnosis of hemostasis disorders. M.: Triada, 2005. 5. Shabalov N.P. Hemorrhagic disorders in newborns / In the book: Neonatology. In 2 vols. / 3rd ed., rev. and additional M.: MEDpress-inform, 2004. T. 2. P. 208–223. 6. Neonatology. National leadership. Krat. ed. / ed. N.N. Volodina. M.: GEOTAR-media, 2013. 7. Takahashi D., Takahashi Y., Itoh S. et al. Late vitamin K deficiency bleeding in an infant born at a maternity hospital // Pediatr Int. 2014 Jun. Vol. 56(3). R. 436. 8. Van Winckel M., De Bruyne R., van de Velde S., van Biervliet S. Vitamin K an update for the pediatrician // Eur J Pediatr. 2009 Feb. Vol. 168(2). R. 127–134. 9. Hubbard D., Tobias JD Intracerebral hemorrhage due to hemorrhagic disease of the newborn and failure to administer vitamin K at birth // South. Med. J. 2006. Vol. 99(11). R. 1216–1220.
Vitamin K deficiency hemorrhagic syndrome in newborns and children in the first months of life
- Narogan Marina Viktorovna
- Karpova Anna Lvovna
- Stroeva Larisa Emelyanovna
Summary
The article is devoted to hemorrhagic disease of the newborn (HDN). Data are provided on the biological role of vitamin K and its metabolism in newborns. The frequency of development, causes and clinical symptoms of early, classic and late forms of the disease are presented. Based on a review of domestic and foreign publications, the issues of laboratory diagnosis, prevention and treatment of GRBN are considered. Considering the danger of life-threatening bleeding, emphasis was placed on the need to maximize the coverage of newborns with prophylactic administration of vitamin K in accordance with Clinical guidelines for the diagnosis and treatment of hemorrhagic disease of newborns, developed by the Association of Neonatologists (2015). We describe a clinical case of the development of a late form of GRBN in a child who was exclusively breastfed and did not receive vitamin K for prophylaxis after birth.
Key words: hemorrhagic disease of newborns, vitamin K Deficiency hemorrhagic syndrome, newborns, vitamin K
Neonatology: news, opinions, training. 2015. No. 3. P. 74-82.
Hemorrhagic disease of newborns (HDN) (ICD-10 code - P53), or vitamin K-deficiency hemorrhagic syndrome, is a disease manifested by increased bleeding in newborns and children in the first months of life due to insufficiency of blood coagulation factors (II, VII, IX, X) , whose activity depends on vitamin K.
The term hemorrhagic disease of the newborn was coined in 1894 (Townsend, 1894) to describe bleeding in newborns not associated with traumatic exposure or hemophilia. Vitamin K deficiency was later shown to be the cause of many of these bleedings, leading to the more accurate term “vitamin K deficiency bleeding” (VKDB) [1].
Biological role of vitamin K and its metabolism in newborns
The biological role of vitamin K is to activate the gamma-carboxylation of glutamic acid residues in prothrombin (factor II), proconvertin (factor VII), antihemophilic globulin B (factor IX) and Stewart-Prower factor (factor X), as well as in plasma antiproteases C and S, playing an important role in the anticoagulation system.
With a lack of vitamin K in the liver, the synthesis of inactive decarboxylated forms of K-dependent factors occurs, which are unable to bind calcium ions and fully participate in blood clotting (PIVKA - protein induced by vitamin K absence or antagonism) [2-4]. Studies usually use the determination of PIVKA-II levels, the decarboxylated form of prothrombin.
In 1929, the Danish biochemist H. Dam isolated a fat-soluble vitamin, which in 1935 was named vitamin K, but to date the metabolic pathways of vitamin K have not been fully studied.
The main source of supply for the body is vitamin K of plant origin, which is called vitamin K1, or phylloquinone. It comes with food - green vegetables, vegetable oils, dairy products. Another form of vitamin K, vitamin K2, or menaquinone, is of bacterial origin. Vitamin K2 is mainly synthesized by intestinal microflora. The role of vitamin K2 has been studied very little. The largest amount of it is located inside bacterial membranes and may be poorly absorbed. Vitamin K2 is not considered to be of much importance to the body. It is known that vitamin K is deposited in the form of menaquinone-4 (MK-4) in the pancreas, salivary glands, and brain. Currently, research is being conducted to study the metabolic pathways of various forms of vitamin K. One of the ways to convert vitamins K1 and K2 into a deposited form is their metabolization in the intestine into an intermediate substance - menadione (vitamin K3). Then, the deposited form of menaquinone-4 is synthesized from menadione circulating in the blood in extrahepatic tissues [4-7].
All newborns have a relative deficiency of vitamin K. The transfer of vitamin K1 across the placenta is extremely limited. The maternal-fetal gradient for vitamin K1 is 30:1, as a result of which the concentration of vitamin K in the blood of the fetus and its reserves at the time of birth are extremely low. Levels of vitamin K1 in umbilical cord blood range from very low (<2 mg/ml) to undetectable. Vitamin K2 is practically not detected in the liver of newborns or occurs in extremely low quantities. This form of the vitamin begins to accumulate gradually during the first months of life. Perhaps, in breastfed children, vitamin K2 accumulates more slowly, since their predominant intestinal microflora ( Bifidumbacterium
,
Lactobacillus
) does not synthesize vitamin K2.
Bacteria that produce vitamin K2 are Bacteroides fragilis
,
E. coli
, are more common in children receiving artificial milk formulas [1, 4, 8].
At the same time, in 10-52% of newborns, an increased level of PIVKA-II is detected in the umbilical cord blood, indicating a deficiency of vitamin K, and by the 3-5th day of life, a high level of PIVKA-II is found in 50-60% of children who are on breastfeeding and who have not received prophylactic administration of vitamin K [4, 9, 10]. Thus, for newborns, the only source of vitamin K is its exogenous supply: with human milk, artificial nutritional formula, or in the form of a drug.
It is known that HRBN develops more often in breastfed children, since the content of vitamin K1 in breast milk is much lower than in artificial milk formulas, usually amounting to <10 μg/l [4]. Whereas artificial milk formulas for full-term babies contain about 50 mcg/l of vitamin K, and formulas for premature babies contain up to 60-100 mcg/l.
Classification of hemorrhagic disease of newborns
There are 3 forms of GRBN depending on the age of manifestation of symptoms: early
,
classical and late
.
The development of bleeding in all forms of the disease is based on vitamin K deficiency. However, the risk factors and causes of the development of symptoms in different forms differ.
Early form of GRBN
Not studied enough. Rarely seen. Manifests during the first 24 hours of a child’s life.
As a rule, the cause of the development of the early form of GRBN is the mother's intake during pregnancy of drugs that interfere with the metabolism of vitamin K, such as indirect anticoagulants (warfarin, phenindione), anticonvulsants (barbiturates, carbamazepine, phenytoin), anti-tuberculosis drugs (isoniazid, rifampicin).
The incidence of this form in children whose mothers received these drugs during pregnancy without vitamin K supplements reaches 6-12% [1, 11]. In general, the frequency of the early form of GRBN, according to 6-year follow-up in Switzerland from 2005 to 2011, was 0.22 per 100 thousand [12].
In the early form, bleeding of any location, including the brain, may occur. Bleeding associated with birth injuries is typical [9, 13]. It is believed that this form of the disease cannot usually be prevented by prophylactic administration of vitamin K after childbirth [1, 14].
Classic form of GRBN
It manifests itself as bleeding on the 2-7th day of life.
In addition to the above reasons that cause vitamin K deficiency in the fetus and newborn, there are 2 more important reasons for the development of this form: 1) lack of prophylactic use of vitamin K immediately after birth and 2) insufficient milk supply.
Gastrointestinal bleeding, skin hemorrhages, bleeding from injection sites/infestations, from the umbilical wound and from the nose are typical. Intracranial hemorrhages are less common [2, 9, 15].
The estimated incidence of the classic form of GRBN without prophylactic use of vitamin K is 0.25-1.5%. Prophylactic administration of vitamin K immediately after the birth of a child can practically eliminate this form of GRBN [1, 12].
Late form of GRBN
It is diagnosed in cases of bleeding symptoms developing in the period from the 8th day to the 6th month of life, although, as a rule, the manifestation occurs at the age of 2-12 weeks [1, 2, 9, 14, 16].
There are 3 main groups of children who are at risk of developing a late form of GRBN.
The first group consists of children with vitamin K deficiency: those who are exclusively breastfed and who have not received vitamin K prophylaxis after birth [12, 14, 17].
Group 2 includes children with impaired absorption of vitamin K in the gastrointestinal tract. This condition is observed in cholestatic diseases and intestinal diseases accompanied by malabsorption (diarrhea for more than 1 week, cystic fibrosis, short bowel syndrome, celiac disease) [1, 18, 19].
Group 3 includes children receiving long-term parenteral nutrition with an inadequate supply of vitamin K.
A feature of the clinical picture of the late form of GRBN is the development of intracranial hemorrhages with a frequency of 30 to 75%, which in 30-50% of cases lead to disability or death [1, 11, 20-22].
Some children experience small “warning” hemorrhages some time before a cerebral hemorrhage (from a day to a week) [16, 17, 23, 24].
Without the prophylactic use of vitamin K immediately after the birth of a child, the incidence of late form of GRBN is in the range of 5-20 per 100 thousand newborns. Intramuscular prophylactic administration of vitamin K can significantly reduce the incidence of the late form, practically eliminating the possibility of its development in children who do not have cholestasis and malabsorption syndrome [1, 12, 25, 26]. In Switzerland, a 6-year follow-up of the development of GRBN from 2005 to 2011. in conditions of three times oral prophylactic intake of a water-soluble form of the vitamin
K (2 mg on the 1st, 4th day and 4 weeks) showed that the frequency of the late form is 0.87 per 100 thousand, while all cases of late bleeding appeared in children who were breastfed and had cholestatic diseases. The development of the classical form has not been registered [12].
Laboratory signs of GRBN
Laboratory signs of GRBN are primarily changes in prothrombin tests: prolongation of prothrombin time (PT), decrease in prothrombin index (PTI), increase in international normalized ratio (INR). A significant change in prothrombin tests is characteristic - 4 times or more. In more severe cases, a prolongation of activated partial thromboplastin time (aPTT) occurs [11, 23, 27, 28].
The levels of fibrinogen, platelets, and thrombin time, as a rule, do not change. However, with massive bleeding and critical conditions, these indicators can become pathological, which is more often observed in the late form of GRBN.
The diagnosis is confirmed by normalization of prothrombin tests and cessation of bleeding after administration of vitamin K [15, 17, 20, 27]. According to Russian authors, complex treatment of the late form of GRBN (administration of menadione and fresh frozen plasma) leads to normalization of prothrombin tests within 6-8 to 18-24 hours [17, 24].
When assessing a coagulogram, it is necessary to take into account that the normative values of hemostasis indicators in newborns and children in the first months of life differ from the reference values in adults and are subject to significant changes immediately after birth. And premature babies have their own characteristics of hemostasis depending on gestational age, characterized by a significant range of values. Newborns and premature infants are characterized by a hypocoagulation orientation of the plasma-coagulation link of hemostasis against the background of increased intravascular coagulation and fibrinolysis activity [increased levels of fibrin degradation products (FDP) and D-dimers] [28-34].
The absolute values of hemostasis parameters depend on the reagent and analyzer, therefore, it is recommended that each laboratory determine its own reference values for newborns and premature infants in accordance with the methodology used [34, 35].
The determination of vitamin K is of no diagnostic value due to its low concentration in newborns [11].
The PIVKA-II level can help in diagnosing hidden vitamin K deficiency, however, it is not considered one of the main diagnostic markers of HDN in practice and is mainly used in scientific works [4, 9].
Treatment of GRBN
It is based on the principles of stopping bleeding and eliminating vitamin K deficiency.
Any child suspected of having GRBN should be given vitamin K immediately, without waiting for laboratory confirmation. In the Russian Federation, the vitamin K preparation is menadione sodium bisulfite (Vicasol), a water-soluble synthetic analogue of vitamin K3. It must be taken into account that its effect begins after 8-24 hours.
In case of ongoing and life-threatening bleeding, the administration of fresh frozen plasma is indicated [2, 9, 36]. Instead of plasma, it is possible to use a concentrated preparation of the prothrombin complex [2, 9, 37]. Its use should be monitored due to the risk of thromboembolic complications [38].
Prevention of GRBN
Prevention of GRBN is a priority for neonatal and pediatric services.
To increase the concentration of vitamin K in the body of a pregnant woman and in breast milk, a woman is recommended to diet using foods rich in vitamin K1, as well as take multivitamin complexes [4, 13, 15].
Pregnant women who take drugs during pregnancy that interfere with the metabolism of vitamin K are recommended to take additional vitamin K: in the third trimester at a dose of 5 mg/day or 2 weeks before birth at a dose of 20 mg/day [1, 14]. However, all these measures are not considered sufficient for the complete prevention of all forms of GRBN.
Taking into account the physiology of the coagulation system and metabolism of vitamin K in newborns, in developed countries, prophylactic administration of a vitamin K drug to all newborns has been accepted, and since the 1960s. Only vitamin K1 preparations are used. Studies conducted up to this time have shown that the drug menadione has an oxidizing effect on fetal hemoglobin, leading to hemolysis, the formation of methemoglobin and Heinz bodies in erythrocytes, which is associated with impaired glutathione metabolism against the background of insufficient antioxidant protection in newborns and, especially, premature infants. The toxic effect of menadione was detected when using high doses (more than 10 mg) [4, 15, 39, 40].
The prophylactic use of vitamin K1 preparations has shown its effectiveness in numerous studies. It is believed that a single parenteral administration of vitamin K1 after the birth of a child is sufficient to prevent the classic and late forms of GRBN in children who do not have symptoms of cholestasis and malabsorption. In some countries, enteral supplementation of vitamin K1 is used for the same purpose, but in these cases it is necessary to take several doses of vitamin K1 orally according to certain regimens. If cholestasis or malabsorption syndrome is present, the child will require additional vitamin K administration [1, 11, 16, 20, 27, 41, 42].
Considering the absence of a vitamin K1 preparation currently registered in the Russian Federation, for the prevention of vitamin K-deficiency hemorrhagic syndrome in our country, intramuscular injection of a 1% solution of menadione sodium bisulfite is used, which is administered in the first hours after birth. During surgical interventions in newborns with possible severe parenchymal bleeding, as well as in children with cholestasis or malabsorption syndrome, additional administration of vitamin K is necessary (see figure).
The effectiveness of the use of menadione can be considered proven for the prevention of the classical form of GRBN in full-term infants, since many studies conducted have obtained identical results: intramuscular administration of menadione (including at a dose of 1 mg) led to a significant increase in PTI, a decrease in APTT, PT, and PIVKA-II, a reduction in the frequency of bleeding, while no toxic effects were registered [27, 29, 43, 44].
The high frequency of intracranial hemorrhages in the late form of hemorrhagic disease in children who are exclusively breastfed makes the prevention of this form especially relevant. Numerous foreign studies have shown the effectiveness of a single parenteral administration of vitamin K1 immediately after the birth of a child for the prevention of this form of the disease. There are practically no studies of the effectiveness of the drug menadione for the prevention of late forms of GRBN in modern literature, which is to some extent explained by what happened in the 1960s. in many countries, replacing it with a vitamin K1 preparation. However, in the domestic literature there are a few publications indicating that the cases of late form of GRBN developed in exclusively breastfed children who did not receive prophylactic administration of the drug menadione in the maternity hospital.
One of the publications presents an analysis of 9 cases of late hemorrhagic disease accompanied by intracranial hemorrhages. The disease developed in children aged from 1 month to 2 months 20 days, who were breastfed and did not have serious somatic pathology. The disease ended unfavorably in 7 (78%) patients: death occurred in 6 children, disability in 1. The authors try to draw attention to the fact that none of the patients received prophylactic administration of vitamin K in the maternity hospital [17].
Another review presents an analysis of 34 cases of late GRBN with intracranial hemorrhage.
The disease manifested itself from the 3rd to the 8th week. All children were breastfed and did not receive vitamin K prophylaxis [24].
Clinical case of late form of GRBN
Boy D
. born from the 3rd pregnancy (1st - frozen, 2nd - full-term birth, the child is healthy), which proceeded without any peculiarities, from 2 births at 39 weeks with a body weight of 2820 g, height 50 cm. Assessment Apgar score was 9/10 points. Attached to the breast in the delivery room. Vaccinated with BCG and hepatitis B vaccine in the maternity hospital. Vitamin K was not administered prophylactically. The patient was discharged home from the maternity hospital in satisfactory condition with a bilirubin level of 200 µmol/l. Was breastfed. In the first month I gained 500 g in weight.
At 2-3 weeks of life, slight umbilical bleeding was noted; he did not receive treatment. At the age of 27 days, there was a slight sanguineous discharge from the nose and a hemorrhagic crust in the nose. The next day, at the age of 28 days, the mother noticed a small hematoma on the child’s back under the shoulder blade, about 1.5 cm in size. In the morning on the 29th day of life, a single vomiting was noted, the child did not suck well, was restless, and drew his legs in. The doctor on duty at the clinic diagnosed intestinal colic.
By evening, the child became lethargic, pale, and was vomiting profusely. On the morning of the 30th day of life, due to progressive deterioration of his condition, he was hospitalized with a diagnosis of prolonged jaundice, intestinal colic, hydrocephalic syndrome.
Upon admission to the hospital. The condition is extremely serious. Body temperature 38 oC. The child practically did not react to the examination. Decortication posture, pronounced hyperesthesia, irritated monotonous cry, bulging of a large fontanelle, anisocoria on the right, the skin was pale icteric in color, a hematoma with a diameter of 1.8-2.0 cm on the back, the subcutaneous fat layer was thinned, and tachycardia was observed. In other organs there are no visible abnormalities.
Survey data
Clinical blood test: Hb 99 g/l, erythrocytes 2.71×1012/l, platelets 165×109/l. In the biochemical blood test: total protein 57 g/l, total bilirubin 227 µmol/l, direct bilirubin 16.1 µmol/l, glucose 5.1 mmol/l, ALT 12 U/l, AST 13.4 U/l.
Coagulogram.
Conclusion: hypocoagulation associated with a deficiency of K-dependent blood coagulation factors (see table).
Based on the anamnesis, clinical picture and additional examination, a diagnosis of “vitamin K deficiency bleeding,” late form, was established.
Stage III intraventricular hemorrhage.” Posthemorrhagic anemia.
For the main diagnosis, treatment was carried out: vikasol 1 mg/kg once a day for 3 days, dicynon, double transfusion of fresh frozen plasma, transfusion of red blood cells.
During treatment, 1 day after admission, coagulogram parameters returned to normal (see table).
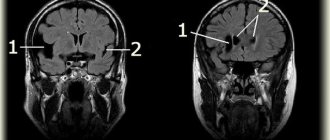
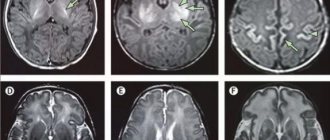
After 1 month, due to the development of occlusive tetraventricular hydrocephalus, the child was transferred to a neurosurgical hospital, where he underwent ventriculoperitoneal shunting.
Conclusion
GRBN is a serious disease that can be fatal or disabling, especially if it develops late. It must be taken into account that the formation of severe intracranial hemorrhages in the late form of GRBN can be prevented by carrying out timely prevention.
The accumulated experience convinces of the need for prophylactic administration of vitamin K preparations to all newborns in the first hours after birth and maintaining vigilance against the late form of GRBN.
In this regard, in 2015, the Association of Neonatologists developed clinical guidelines for the diagnosis and treatment of GRBN. A regimen for the prevention of GRBN has also been proposed [36]. Unfortunately, in the Russian Federation, 100% routine prevention of HDN is difficult to implement, since hemolytic disease in a newborn is an official contraindication for the administration of the only vitamin K drug registered in our country - menadione; its appointment in this group of children is possible only if there are serious arguments (see figure).
Prevention of the late form of GRBN should include the administration of vitamin K in the maternity hospital and maintaining vigilance against this disease in children in the first six months of life from high-risk groups: those who are breastfed, those with cholestasis syndrome and malabsorption syndrome. In this regard, the appearance of hemorrhages in children in the first months of life requires immediate differential diagnosis and exclusion of vitamin K-deficient bleeding.
Such warning hemorrhages include:
✧ nosebleeds;
✧ bleeding from the umbilical wound;
✧ petechiae and ecchymoses on the skin or mucous membranes;
✧ intermuscular hematomas or bleeding from sites of invasive interventions (injections, vaccinations, blood sampling sites, circumcision, operations).
If the development of GRBN is suspected, immediate administration of the drug menadione is indicated to avoid the development of life-threatening bleeding.
After registration of a vitamin K1 preparation in Russia, clinical recommendations for the prevention and treatment of vitamin K-deficiency bleeding in children will be revised and the use of vitamin K1 preparations will be recommended.
LITERATURE
1. NHMRC (National Health and Medical Research Council) (2010). Joint statement and recommendations on Vitamin K administration to newborn infants to prevent vitamin K deficiency bleeding in infants - October 2010 (the Joint Statement). Commonwealth of Australia.www.ag.gov.au/cca. ISBN Online: 1864965053.
2. Neonatology. National leadership. Brief edition / ed. acad. RAMS N.N. Volodina. M.: GEOTAR-Media, 2013. 896 p.
3. Joshi A., Jaiswal JP Deep vein thrombosis in protein S deficiency // J. Nepal. Med. Assoc. 2010. Vol. 49. P. 56-58.
4. Greer FR Controversies in neonatal nutrition: macronutrients and micronutrients. In: Gastroenterology and nutrition: neonatology question and controversies. 2nd ed. by Neu J. Philadelphia: Elsevier saunders, 2012. P. 129-155.
5. Card DJ, Gorska R. et al. Vitamin K metabolism: Current knowledge and future research // Mol. Nutr. Food Res. 2014. Vol. 58. P. 1590-1600.
6. Thijssen KHW, Vervoort LMT et al. Menadione is a metabolite of oral vitamin // Br. J. Nutr. 2006. Vol. 95. P. 260-266.
7. Sharer MJ, Newman P. Recent trends in the metabolism and cell biology of vitamin K with special reference to vitamin K cycling and MK-4 biosynthesis // J. Lipid Res. 2014. Vol. 55, N 3. P. 345-362.
8. Thureen PJ, Hay WW Neonatal Nutrition and Metabolism. 2nd Ed. Jr. Cambridge University Press. 2006.
9. Gomella TL Neonatology: Management, Procedures, On-Call Problems, Diseases, and Drugs. McGraw-Hill. 2013.
10. von Kries R., Kreppel S., Becker A., Tangermann R., Gobel U. Acarboxyprothrombin concentration (corrected) after oral prophylactic vitamin K // Arch. Dis. Child. 1987. Vol. 62. P. 938-940.
11. Nimavat DJ Hemorrhagic Disease of Newborn. Updated: Sep 26, 2014. https://emedicine.medscape.com/article/974489-overview.
12. Laubscher B., Banziger O., Schubiger G., the Swiss Paediatric Surveillance Unit (SPSU). Prevention of vitamin K deficiency bleeding with three oral mixed micellar phylloquinone doses: results of a 6-year (2005-2011) surveillance in Switzerland // Eur. J. Pediatr. 2013. Vol. 172. P. 357-360.
13. Shearer MJ Vitamin K deficiency bleeding (VKDB) in early infancy // Blood. Rev. 2009. Vol. 23. P. 49-59.
14. Burke CW Vitamin K Deficiency Bleeding // J. Pediatr Health Care. 2013. Vol. 27, N 3. P. 215-221.
15. Shabalov N.P. Neonatology. 5th ed., rev. and additional, in 2 volumes. M.: MEDpress-inform, 2009. 1504 p. (in Russian)
16. Schulte R., Jordan LC, Morad A., Naftel RP, Wellons JC, Sidonio R. Rise in late onset vitamin K deficiency bleeding in young infants because of omission or refusal of prophylaxis at Birth. Pediatric Neurology. 2014. Vol. 50. P. 564-568.
17. Lobanov A.I., Lobanova O.G. Hemorrhagic disease of newborns with late onset. Issues of modern pediatrics. 2011. No. 1. P. 167-171.
18. Feldman AG, Sokol RJ Neonatal cholestasis // Neoreviews. 2013. Vol. 14, N 2. e63.
19. van Hasselt PM, de Koning T.J., KvistN. et al. Prevention of Vitamin K Deficiency Bleeding in Breastfed Infants: Lessons From the Dutch and Danish Biliary Atresia Registries. Pediatrics. 2008. Vol. 121, N 4. e857-e863.
20. Notes from the field: late vitamin K deficiency bleeding in infants whose parents declined vitamin K prophylaxis // Tennessee. MMWR Morb Mortal Wkly Rep. 2013. Vol. 15, N 62 (45). P. 901-902.
21. Volpe JJ Neurology of the Newborn. 5th ed. Philadelphia: Elsevier, 2008. 1094 p.
22. Volpe JJ Intracranial Hemorrhage in Early Infancyd Renewed Importance of Vitamin K Deficiency // Pediatric Neurology. 2014. Vol. 50. P. 545-6.
23. Ursulenko E.V., Martynovich N.N., Tolmacheva O.P., Ovanesyan S.V. A case of late hemorrhagic disease in a 6-week-old child, complicated by the development of acute cerebrovascular accident and hemothorax // Siberian Medical Journal. 2012. No. 2. P. 114-118.
24. Lyapin A.P., Kasatkina T.P., Rubin A.N. and others. Intracranial hemorrhages as a manifestation of late hemorrhagic disease of newborns // Pediatrics, 2013. No. 2. P. 38-42.
25. Cornelissen M., Von Kries R., Schubiger G., Loughnan PM. Prevention of vitamin K deficiency bleeding: efficacy of different multiple oral dose schedules of vitamin K // Eur. J. Pediatr. 1997. Vol. 156, N 2. P. 126-130.
26. Von Kries R. Oral versus intramuscular phytomenadione: Safety and efficacy compared // Drug Safety. 1999. Vol. 21, N 1. P. 1-6.
27. Wariyar U., Hilton S., Pagan J., Tin W., Hey E. Six years' experience of prophylactic oral vitamin K // Arch. Dis. Child Fetal Neonatal. 2000. Vol. 82, N 1. F64-F68.
28. Puckett RM, Offringa M. Prophylactic vitamin K for vitamin K deficiency bleeding in neonates // Cochrane Database of Systematic Reviews. 2000. Is. 4, N CD002776.
29. Chuprova A.V. The system of neonatal homeostasis in normal and pathological conditions (scientific review) // Bulletin. SO RAMS. 2005. No. 4 (118). pp. 13-19.
30. Shabalov N.P., Ivanov D.O. Shabalova N.N. Hemostasis in the dynamics of the first week of life as a reflection of the mechanisms of adaptation to extrauterine life of a newborn // Pediatrics. 2000. N 3. P. 84-91.
31. Andrew M., Paes B., Milner R., et al. Development of the human coagulation system in the full-term infant // Blood. 1987. Vol. 70. P. 165-172.
32. Andrew M., Paes B., Milner R. et al. Development of the human coagulation system in the healthy premature infant // Blood. 1988. Vol. 72. P. 1651-1657.
33. Mitsiakos G., Papaioannou G. et al. Haemostatic profile of full-term, healthy, small for gestational age neonates // Thrombosis Research. 2009. Vol. 124. P. 288-291.
34. Motta M., Russo FG Developmental haemostasis in moderate and late preterm infants // Ital. J. Pediatr. 2014. 40 (Suppl 2): A38.
35. Dorofeeva E.I., Demikhov V.G. and others. Features of hemostasis in newborns // Thrombosis, hemostasis and rheology. 2013. No. 1 (53). pp. 44-47.
36. Monagle P., Massicotte P. Developmental haemostasis: Secondary haemostasis // Seminars in Fetal & Neonatal Medicine. 2011. Vol. 16. P. 294-300.
37. Degtyarev D.N., Karpova A.L., Mebelova I.I., Narogan M.V. and others. Draft clinical recommendations for the diagnosis and treatment of hemorrhagic disease of newborns // Neonatology, 2015. No. 2. P. 75-86.
38. Krastaleva I.M., Shishko G.A. and others. Problems of treatment of hemorrhagic disease in newborns // Medical news. 2014. No. 9 (240). pp. 60-62.
39. Alarcon P., Werner E., Christensen RD Neonatal hematology pathogenesis, diagnosis, and Management of Hematologic Problems 2nd Edition // Cambridge University Press. 2013.
40. Report of Committe on Nutrition: vitamin K compounds and the water-soluble analogues // pediatrics. 1961. Vol. 28. P. 501-507.
41. Shahal Y., Zmora E., Katz M., Mazor D., Meyerstein N. Effect of vitamin K on neonatal erythrocytes // Biol. Neonate. 1992. Vol. 62. N 6. P. 373-8.
42. Ipema HJ Use of oral vitamin K for prevention of late vitamin K deficiency bleeding in neonates when injectable vitamin K is not available // Ann. Pharmacother. 2012. Vol. 46. P. 879-883.
43. Takahashi D., Shirahata A., Itoh S., Takahashi Y. et al. Vitamin K prophylaxis and late vitamin K deficiency bleeding in infants: Fifth nationwide survey in Japan // Pediatric. International. 2011. Vol. 53. P. 897-901.
44. Chawla D., Deorari AK, Saxena R., Paul VK et al. Vitamin K1 versus vitamin K3 for prevention of subclinical vitamin deficiency: a randomized controlled trial // Indian. Pediatr. 2007. Vol. 44, N 11. P. 817-822.
45. Dyggve HV, Dam H., Sondergaard E. Comparison of the action of vitamin K1 with that of synkavit in the newborn // Acta Paediatrica. 1954. Vol. 43. N 1. P. 27-31.
Hemorrhagic disease of the newborn (HDN) is a disease of children of the neonatal period, manifested by increased bleeding due to insufficiency of blood coagulation factors, the activity of which depends on the content of vitamin K in the body. Hemorrhagic disease was first described in 1893 by Chartes Townsend. The term was originally used to distinguish hemorrhagic conditions observed only in the neonatal period (primarily the 1st week) from hemorrhagic conditions that last a lifetime, such as hemophilia [13].
Late HDN is a disease of children in the first 512 weeks of life due to nutritional deficiency of vitamin K. Insufficient intake of this vitamin in breast milk and various concomitant diseases of the child contribute to the disruption of the synthesis of “coagulologically” active blood clotting factors in his body, which in some cases leads to hemorrhagic complications, including cerebral hemorrhages.
Late HDN was first identified in the 70s of the last century. In various countries, pediatricians drew attention to the fact that in children who are breastfed (usually in the first 3 months of life), many diseases are more often complicated by increased bleeding than in infants who are bottle-fed. The incidence of late HDN ranges from 4 to 10 cases per 10,000 newborns (in England 1:1200, Japan 1:1700, Thailand 3:1200) [10].
Among the concomitant conditions or diseases during the newborn period, against the background of which the passage of vitamin K in the child’s body is most often disrupted, there are: violation of bacterial colonization of the gastric tract, especially due to long-term use of antibacterial drugs by the mother or child, fermentopathy, malabsorption syndrome. Violation of the entry of vitamin K into the systemic bloodstream of an infant and its metabolism is facilitated by hypoxia, prematurity and immaturity of the infant, conditions leading to disruption of hepatic blood flow, the consequences of intrauterine infection, hepatitis, biliary atresia with outcome in cirrhosis of the liver, hepatocellular diseases [11, 12].
The purpose of this study: to study the features of clinical and laboratory manifestations of blood coagulation disorders, to determine methods for the prevention and treatment of children with late TTH.
A retrospective analysis of the medical records of 29 children admitted for treatment to the Republican Scientific and Practical Center for Pediatric Oncology, Hematology and Immunology of the Ministry of Health of the Republic of Belarus in 2001-2012 was carried out. regarding late HDN. The age of the newborns was 415 weeks, body weight 4.14.9 kg. The results of general clinical, biochemical and coagulological studies were analyzed.
After administration of a concentrate of non-activated factors of the prothrombin complex, bleeding in all children was stopped within 1560 minutes. The drug of choice for the correction of blood coagulation disorders is a concentrate of non-activated vitamin K-dependent coagulation factors (II, VII, IX and X in combination with protein C and protein S).
The introduction into practical healthcare of measures to prevent nutritional deficiency of vitamin K by taking multivitamin complexes containing vitamin K by a nursing mother will avoid the development of late hemorrhagic disease with subsequent disability in newborns by preventing hemorrhagic complications.
LITERATURE 1. Greenberg CS, Orthner CL // Wintrobes Clinical Hematology. Philadelphia, 1997. Vol. 1. P. 685-764. 2. Hougie C., Barrow EM, Graham JB // J. Clin. Invest. 1957. Vol. 6, No. 3. P. 485-496. 3. Reverdiau-Moalic P., Delahousse B., Body G., et al. // Blood. 1996. Vol. 88. P. 900-906. 4. Dam H. // Nature. 1935. Vol. 135. P. 652. 5. Kukhta V.K., Morozkina T.S., Oletsky E.I., Taganovich A.D. Biological chemistry: Textbook / Ed. A. D. Taganovich. Minsk, M., 2008. 6. Sutor A. // Thromb. Haemost. 1999. Vol. 81, No. 3. P. 456-461. 7. Widdershoven J., van Munster P., de Abreu R., et al. // Clin. Chem. Nov. 1987. Vol. 33, No. 11. P. 2074-2078. 8. Andrew M., Vegh P., Johnston M., et al. // Blood. 1992. Vol. 80, No. 8. P. 1998-2005. 9. Practical Hemostasis and Thrombosis / Ed. D. OShaughnessy, M. Makris, D. Lillicrap. Blackwell Publishing, 2005. 10. Pichler E., Pichler L. // Wien Med. Wochenschr. 2008. Bd. 158. S. 385-395. 11. Andrew M., Paes B., Milner R., et al. // Blood. 1987. Vol. 70, No. 1. P. 165-170. 12. Lanzkovsky F. Children's oncology and hematology. M., 2005. 13. Booth SL, Suttie JW // J. Nutr. 1998. Vol. 128, No. 5. P. 785-788. 14. Godier A., Susen S., Samama CM // J. Thromb. Haemost. 2010. Vol. 8. P. 2592-2595. 15. Rumyantsev A. G., Maschan A. A., Samochatova E. V. Accompanying therapy and control of infections in hematological and oncological diseases. M., 2006. 16. Porretti L. // Blood Transfus. 2012. Vol. 10. P. 351-359. 17. Land WG // Transfus. Med. Hemother. 2013. Vol. 40. P. 313. 18. Beckers EAM // Transfusion. 2011. Vol. 51. P. 1278-1283. 19. Honickel M., Rieg A., Rossaint R., et al. // Thromb. Haemost. 2011. Vol. 106, No. 4. P. 724-733.
Received 11/28/13.
Address for correspondence: Dmitriev Vyacheslav Vasilievich.
Republican Scientific and Practical Center for Pediatric Oncology, Hematology and Immunology. 223053, Minsk district, Borovlyany village, st. Frunzenskaya, 43; sl. tel. (8-017) 265-42-22. Key words:
diagnosis, treatment, newborns, late hemorrhagic disease, prevention, blood coagulation
Author(s):
Dmitriev V.V., Dmitriev E.V.
Medical institution: “Republican Scientific and Practical Center for Pediatric Oncology, Hematology and Immunology of the Ministry of Health of the Republic of Belarus”