Home » Department of Cardiology and Cardiac Surgery » Diseases » Ebstein's anomaly
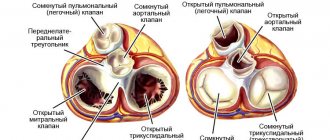
Ebstein's anomaly is a fairly rare congenital heart defect (CHD), which is characterized by displacement of the tricuspid (tricuspid) valve leaflets into the cavity of the right ventricle. That is, the leaflets of the pathologically altered valve are formed from the walls of the right ventricle (RV), and not from the atrioventricular (atrioventricular) ring, as is normal, and they do not close completely, so tricuspid valve insufficiency develops.
This defect accounts for about 1% of all congenital heart defects. In addition, 75% of people with this pathology also have an atrial septal defect. Also quite common are pathological pathways through which abnormal conduction of impulses occurs. This is the cause of the development of arrhythmias. Ventricular arrhythmias are the most common. They and congestive heart failure are the main causes of death in patients with this pathology. If the defect is allowed to develop naturally, 60% of patients die by the age of 20.
Types of Ebstein's anomaly
There are 4 types of Ebstein's anomaly:
- Type I - the posterior and septal leaflets are displaced or absent, the anterior valve leaflet is mobile and large.
- Type II - the anterior, septal and posterior leaflets are usually present, but they are shifted towards the apex of the heart and are relatively small.
- Type III - the anterior valve leaflet has shortened and fused chords, restrictions in movement and is often shortened. The posterior and septal valve leaflets are underdeveloped and displaced.
- Type IV - the anterior leaflet of the valve is severely deformed and also displaced in the direction of the outflow tract from the right ventricle. The posterior valve is usually underdeveloped or absent, and the septal valve is represented by fibrous tissue.
Ebstein's anomaly: cone reconstruction surgery - the first experience of anatomical correction
The most complete first coverage of the features of Ebstein’s anomaly in Russian literature was given by R.P. Zubarev in the book “Ebstein’s Anomaly” [1].
Currently, a large amount of material has been accumulated on the embryogenesis of this congenital heart defect (CHD). The mechanisms causing disruption of valve separation have not been established. However, there is evidence of the frequent occurrence of this defect with mutations of the NKX2.5, 10p13-p14, 1p34.3-p36.11 gene and in women who took benzodiazepines or drugs containing lithium in early pregnancy [2]. There are known familial cases of this defect [3]. We have two such patients under observation. In one case, these are identical twins, since the mother implanted one egg through in vitro fertilization, and in the other case, these are monochorionic twins. In one child, Ebstein's anomaly was combined with a ventricular and atrial septal defect and pulmonary artery atresia, and in another, with an atrial septal defect. The second children do not have congenital heart defects. The genetic material in these cases is identical, as is the nature of the influence of environmental factors.
The anatomical features of this pathology are described in detail by N.A. Belokon and V.P. Podzolkov (1990) [4]. It should be noted that this congenital defect has pronounced individual variability. The main anatomical feature of the defect is the displacement of the tricuspid valve into the cavity of the right ventricle towards the apex of the heart, usually to the junction of its inflow and trabecular parts [5, 6]. The degree of dysplasia, deformation of the valves and their structures varies widely:
- the process of delamination (separation) of the tissue of the tricuspid valve leaflets from the endocardium of the right ventricle is disrupted (Fig. 2);
- the posterior and septal leaflets are spread across the endocardium of the right ventricle, and the free parts of the leaflets are displaced apically from the valve ring;
- the chordal apparatus of the posterior and septal valves is shortened or absent;
- there is a shift in the anteropical direction of the level of cooptation of the tricuspid valve leaflets;
- the anterior leaflet of the tricuspid valve compensatory enlarges, thickens, and fenestrations often appear in it;
- the zone of the true tricuspid ring expands;
- dilatation of the atrialized part of the right ventricle occurs with thinning of the myocardium in it and replacement of the latter by fibrosis;
- the cavity of the right atrium increases significantly.
In 90% of patients with Ebstein's anomaly under observation, an atrial septal defect was detected. One patient had pulmonary atresia, and two patients had mitral valve regurgitation. Additional pathways for conducting electrical impulses from the atria to the ventricles were recorded in 28.5% of patients.
The natural history of patients with Ebstein's anomaly is unfavorable. According to a study by A. Yetman et al. (1997), who analyzed the survival of 46 newborn patients with Ebstein's anomaly and cyanosis, 70% of patients died. According to the cardiac surgery department of Children's Hospital No. 1 in St. Petersburg, one woman was diagnosed with frozen pregnancy of a fetus that was observed with a diagnosis of Ebstein's anomaly, and two children with Ebstein's anomaly died in the first month of life. A child with a severe form of Ebstein's anomaly was observed at the Samara Cardiac Dispensary. The child died suddenly at home at the age of 7 months.
In connection with the emergence of the possibility of anatomical correction of this heart defect using one’s own tissues at an early age, performing a cone reconstruction operation, it can be argued that a radical change has appeared in solving this problem. An important problem remains the ability to identify patients whose risk of sudden death exceeds the risk of undergoing surgical treatment at an early age.
Materials and research methods
This study was conducted based on the analysis of clinical material from 2014 to the present. The results of surgery for Ebstein's anomaly in three cardiac surgery centers were taken into account. The clinical characteristics of the patients are shown in Table.
results
In patients operated on before the age of 5 years, various complications were observed with a high frequency, including: reciprocal atrioventricular (28.5%) and non-reciprocal atrial rhythm disturbances (33.3%), the presence of concomitant heart defects and hemodynamic decompensation .
Among them was a patient aged 11 months. The child was admitted to the hospital for emergency reasons due to severe thrombocytopenic purpura. Chronic hypoxemia led to decompensation of DIC syndrome. During surgery, a flat, organized thrombus was identified and removed in this patient's right ventricular cavity.
Another patient was operated on for Ebstein's anomaly on the second day of life. The child's body weight was 2 kg 300 g. He was the second of twins. The first child is healthy. The operation was required for emergency reasons due to the presence of pulmonary atresia in the child and hemodynamics, depending on the functioning of the ductus arteriosus. The child underwent anatomical correction of Ebstein's anomaly under artificial circulation, the atrial septal defect and ventricular septal defect were closed, and the pulmonary artery was reconstructed with a venous valve-containing homograft. According to the literature, such a successful correction was performed for the first time in the world.
The third child had progressive pulmonary hypertension secondary to a large ventricular septal defect.
The fourth patient was 5 years old. His surgery was performed for urgent reasons due to acutely developed bradyarrhythmia (heart rate 36–48 beats per minute) 2 months after repeated radiofrequency ablation. Radiofrequency ablation was performed for accessory pathways leading to persistently recurrent atrioventricular reciprocal hemodynamically significant tachycardia. He developed complete atrioventricular block. During the operation, he was implanted with a permanent dual-chamber pacemaker, and after 2 months, according to Holter monitoring, he had correct sinus rhythm 86% of the time. The results are summarized in Fig. 1.
When analyzing the indications for surgical treatment of Ebstein's anomaly at an early age, the following main links of hemodynamic disturbances identified in all operated patients were identified:
- reduction of the right ventricular cavity due to a shift in the level of cooptation of the valves to the apex of the heart, leading to impaired diastolic function of the right ventricle;
- regurgitation on the tricuspid valve caused volume overload of the right heart and led to progressive dilatation of the atrialized part of the right ventricle and right atrium when observed over time;
- the atrialized part of the right ventricle ejects blood in a retrograde direction, increasing the volume overload of the right parts of the heart;
- tachyarrhythmias disrupted compensation mechanisms, causing acute cardiovascular failure.
These hemodynamic disturbances are interrelated, progress over time, and when rhythm disturbances occur, they are life-threatening. In this regard, the risk of delaying surgical treatment exceeds the risk of performing surgery in young children.
The progressive replacement of the muscle tissue of the right ventricle in the atrialized part with fibrosis plays a significant role in the pathogenesis of the disease. Based on the surgical material, morphological preparations presented in Fig. 1 were obtained. 2, 3 and 4.
This specimen shows pronounced thickening of the endocardium. Pronounced signs of fibrosis in the endomysium and sclerotic changes in the thinned myocardium.
All preparations show pronounced replacement of the myocardial connective tissue matrix.
A convincing example of morphological changes in the atrialized part of the right ventricle is an operational photograph that characterizes the native appearance of the atrialized wall of the right ventricle after separation of the posterior leaflet of the tricuspid valve. This is what the myocardium of the inflow tract of the right ventricle looked like in all patients (Fig. 5).
The wall of the atrialized part of the right ventricle after separation of the leaflets is a layer of epicardium and myocardium 2–3 mm thick. Its functionality is questionable. The risk of developing an aneurysm and the risk of circular electric currents occurring in it is quite high.
Summarizing the anatomical changes that cause hemodynamic disturbances and morphofunctional changes in the cavity of the right ventricle, we can conclude that patients with a severe form of Ebstein’s anomaly should be operated on in early childhood, can be operated on in adolescence, and not all adult patients with Ebstein’s anomaly will be able to undergo surgery to radically correct a heart defect.
Nineteen patients with Ebstein's anomaly underwent cone reconstruction.
In all patients, the operation was performed on a stopped heart. The average aortic cross-clamp time was 127 ± 34 minutes. Cardioplegia was performed with Custodiol solution. Cardioplegia was repeated after 90–110 minutes. Hypothermia during correction was 28–32 °C.
The operation diagram is shown in Fig. 6.
The purpose of this operation is to create a cone from the tissues of the tricuspid valve without pulling the leaflets to the fibrous ring. It is important that the mobility and windage of the valves be maintained, and that the papillary muscles of the notochordal apparatus be as close to each other as possible. In 7 patients, the marginal chords of the posterior and septal leaflets, and the two and anterior leaflets of the tricuspid valve were absent. The chordae were created after separating the leaflets by making cuts 1/4 of the length of the leaflets, from the edge of their attachment to the myocardium. The essential point of this operation was the movement of the posterior leaflet 180 degrees clockwise to the septal leaflet. Its mobility was ensured by cutting off the apical edge of the valve from the myocardium and re-fixing it to areas of the septal valve displaced to the apex of the heart or directly to the endocardium of the right ventricle. The need for excision of the atrialized part of the right ventricle or its plication remains a controversial issue. In this study, in 7 out of 19 patients, including a newborn child, the atrialized part of the myocardium in the intervascular zone was excised. It was in these patients that the best restoration of the shape of the inflow portion of the right ventricle was achieved. It should be noted that in 8 patients this was impossible due to the scattered type of coronary arteries. Plication of the atrialized part of the right ventricle was ensured by U-shaped sutures with large velor pads to prevent cutting of the sutures. This risk is due to thinning and impaired myocardial strength in the atrialized part of the right ventricle. All patients underwent narrowing of the annulus fibrosus. In two patients, narrowing of the annulus fibrosus was performed at the “3” and “10” o’clock levels. All patients required suturing of the holes in the valve leaflets to create a cone. Of great importance is the creation of support for the posterior valve thanks to additional fixing sutures between the posterior and septal valves, which provide the role of opening the “sail”.
In 16 patients, the tricuspid valve was reimplanted into the annulus fibrosus with a continuous enveloping suture. According to the presented material, a continuous suture in the area of the atrioventricular node was carried out with an “exit” to the right atrium. In one patient, a dual-chamber pacemaker with two epicardial electrodes was immediately implanted, since he was operated on urgently due to the development of complete atrioventricular block two months after repeated radiofrequency ablation. This patient had continuously recurrent atrioventricular reentrant tachycardia for 4 years.
In the early postoperative period, 16 patients required dopamine and 4 patients required a short course of 1–5 days of adrenaline.
One patient required surgery to create a bidirectional cavopulmonary anastomosis on the fourth day.
The follow-up time for patients after surgery ranged from 2 months to 3.5 years. On average 1.8 ± 0.74 years. All patients underwent ultrasound examination every 3–6 months. An important prognostic sign is effective reconstruction of the inflow tract of the right ventricle: suturing or resection of the atrialized part of the right ventricle. Bringing papillary muscles or chordae attachment sites closer together.
Three months after discharge, only 6 patients were receiving angiotensin-converting enzyme inhibitors. All patients maintained high exercise tolerance. According to dynamic monitoring of the electrocardiogram, no relapses of paroxysmal changes were detected in the group of operated patients.
It should be noted that the anatomy of Ebstein's anomaly is always individual. In connection with this, the decision on the nature of treatment is always made individually. The cone reconstruction operation successfully corrects anatomical disorders and is also always of a strictly individual nature. As experience and clinical material accumulate, this operation should become part of the main arsenal of pediatric and adult cardiac surgery departments.
conclusions
- Cone reconstruction is an operation that allows you to perform anatomical correction of congenital heart disease - Ebstein's anomaly at any age.
- If life-threatening complications occur, this operation can be performed at an early age, including newborns.
- Based on the results obtained, this operation can also be performed in older people, with mandatory consideration of the contractile function of the right and left ventricles.
Literature
- Zubarev R.P. Ebstein's anomaly. M.: Medicine, 1975. 112 p.
- Park JM Ebsten's anomaly of the tricuspid valve associated with prenatal exposure to lihium carbonate // Amer. J. Dis. Child. 1980. Vol. 134. No. 7. P. 703–704.
- Attenhofer Jost CH, Connolly HM, Dearani JA, Edwards WD, Danielson GK Ebstein's anomaly. Circulation. 2007. 115 (2): 277–285. doi:10.1161/ CIRCULATIONAHA.106.619338. PMID 17228014.
- Belokon N. A., Podzolkov V. P. Congenital heart defects. M.: Medicine, 1990. 352 p.
- Dearani JA, Bacha E., da Silva JP Cone Reconstruction of the Tricuspid Valve for Ebstein's Anomaly: Anatomic Repair.
- Reddin G, Poterucha JT, Dearani JA, Warnes CA et al. Cone Reconstruction of Atypical Ebstein Anomaly Associated with Right Ventricular Apical Hypoplasia // Tex Heart Inst J. 2021 Feb; 43(1):78–80. DOI: 10.14503/THIJ-15–5011.
V. A. Bolsunovsky*, 1, Candidate of Medical Sciences G. G. Khubulava**, Doctor of Medical Sciences, Professor, Academician of the Russian Academy of Sciences G. A. Novik*, Doctor of Medical Sciences, Professor M. V. Zhdanova*, Candidate of Medical Sciences R. R. Movsesyan***, Doctor of Medical Sciences, Professor, Corresponding Member of the Russian Academy of Sciences S. E. Shorokhov****, Doctor of Medical Sciences M. S. Khokhlunov**** A. V. Bolsunovsky*
* Federal State Budgetary Educational Institution of Higher Education St. Petersburg State Pediatric Medical University of the Ministry of Health of the Russian Federation, St. Petersburg ** Federal State Budgetary Educational Institution of Higher Education Military Medical Academy named after. S. M. Kirova Ministry of Defense of the Russian Federation, St. Petersburg *** St. Petersburg State Budgetary Healthcare Institution Children's City Hospital No. 1, St. Petersburg **** State Budgetary Healthcare Institution SOKKD, Samara
1 Contact information
Causes of development of Ebstein's anomaly
As is known, in the prenatal period, the human heart is formed in the first 9 weeks of pregnancy. It is at this time that all congenital heart defects develop.
It is during this period that Ebstein's anomaly is determined. This happens under the influence of a number of factors:
- viral infections;
- scarlet fever;
- measles;
- rubella;
- use of certain medications (sedatives, hypnotics, lithium preparations);
- drugs;
- alcohol.
Family history also plays an important role. It should be noted that the above reasons cause not only Ebstein's anomaly, but also almost all other congenital heart defects.
Causes and risk factors
It is assumed that Ebstein's anomaly is caused by mutations in the CFA9 locus of chromosome 17q, duplication of chromosome 15q, and a defect in the ALK3/BMPR receptor. Chromosomal abnormalities occur at the stage of fusion of parental genetic material or in the early stages of pregnancy and lead to incorrect formation of organs and tissues of the child’s body in the prenatal period.
Since the exact causes of the disease have not yet been established, the most likely risk factors are:
- maternal use of lithium during pregnancy;
- maternal use of benzodiazepines during pregnancy;
- viral diseases suffered in the early stages of pregnancy (influenza, rubella, measles);
- history of multiple spontaneous abortions in the early stages;
- chronic intoxication with pesticides, vapors of paints and varnishes, petroleum products, etc. (living in environmentally unfavorable areas, working in hazardous industries);
- parental use of illegal substances, alcohol abuse, smoking.
Viral diseases in early pregnancy can lead to the development of Ebstein's anomaly in the fetus
Pathophysiology of Ebstein's anomaly
Due to the fact that the valve leaflets are formed from the tissues of the ventricle and are localized in it, a significant reduction in its cavity occurs. According to the decrease in the atrium cavity, the volume of blood ejected by the heart into the pulmonary trunk also decreases.
We should also not forget that the valve leaflets have an abnormal shape and are not able to completely isolate the ventricular cavity from the atrium cavity. There is a reflux of blood into the atrium, which causes stagnation of blood in the atrium and its hypertrophy. And since this is often accompanied by a septal defect, blood is dumped into the left atrium, which leads to mixing of arterial and venous blood and a decrease in blood oxygen saturation.
Treatment
The main method of radical elimination of Ebstein's anomaly is surgery, which can be carried out in one or several stages.
90% of patients with Ebstein's anomaly have positive immediate and long-term results after surgical treatment.
Indications for surgical treatment:
- heart failure FC III–IV (functional classes);
- significant or progressive cyanosis - the level of blood oxygen saturation (saturation index) is less than 80%;
- severe cardiomegaly with a cardiothoracic index greater than 0.65;
- concomitant cardiac anomalies;
- atrial and ventricular arrhythmias;
- a history of paradoxical embolism.
Reconstructive operations include correction of the tricuspid valve, its relocation, replacement (prosthetics), closure of the atrial septum, and plastic surgery of the atrialized part of the right ventricle.
Surgical intervention is the main treatment method for Ebstein's anomaly
Surgical treatment improves survival, prognosis, prevents the development or significantly reduces the severity of arrhythmias.
Symptoms of Ebstein's anomaly
The clinical symptoms of Ebstein's anomaly directly depend on the degree of tricuspid valve insufficiency, the type of heart rhythm disturbance, the amount of blood discharged through the atrial septal defect, as well as the degree of narrowing of the outflow tract of the RV (right ventricle).
Newborns with types III and IV Ebstein's anomaly develop severe hypoxemia after closure of the ductus arteriosus (patent ductus arteriosus).
- Patients begin to complain of shortness of breath, increased fatigue, pain in the heart, palpitations (feeling of heart contractions).
- The skin is bluish (cyanotic).
- Swelling appears in the lower extremities (a consequence of venous stagnation).
- In patients, the terminal phalanges of the arms and legs change (the appearance of “watch glasses” and “drumsticks”).
All these symptoms indicate that the body tissues are experiencing a lack of oxygen.
Diagnostics
An objective examination of the cardiovascular system determines:
- expansion of the boundaries of cardiac dullness to the right and left;
- dull, weakened heart sounds; a gallop rhythm is often heard, that is, a three- or four-part rhythm caused by the bifurcation of the first and second heart sounds or the presence of additional third and fourth sounds.
Ebstein's anomaly is extremely rare: it accounts for no more than 1 case out of 100 (according to some sources - 200) congenital heart defects.
The data from instrumental research methods are as follows:
- with a low-voltage ECG - pronounced pointed P waves, which indicate hypertrophy and dilatation of the right atrium, blockade of the right bundle branch, signs of Wolff-Parkinson-White syndrome (WPW);
- X-ray examination shows cardiomegaly, decreased intensity of the pulmonary pattern; in the lateral projection, abnormal filling of the retrosternal space;
- with an ultrasound examination of the heart - lengthening, thickening and sagging of the petals of the tricuspid valve, expansion of the right atrium, with Doppler ultrasound - a characteristic “howling” sound of movement of the leaflets;
- during cardiac catheterization (performed in rare cases) - increased pressure in the right atrium;
- angiocardiography reveals a gigantic, sharply dilated cavity of the right atrium with high contrast intensity.
X-ray of Ebstein's anomaly
Diagnosis of Ebstein's anomaly in Israel
- Auscultation reveals dull first and second sounds.
- X-ray : an increase in the size of the right atrium, depletion of pulmonary blood flow, the cardiothoracic index often approaches one.
- ECG (electrocardiography) : signs of right atrium hypertrophy are noted.
- EchoCG (echocardiography).
- Fetal echocardiography (EchoCG) : the defect is diagnosed prenatally (during pregnancy) in 60% of cases. The main symptom is a pronounced increase in heart size, mainly due to the right atrium. On the tricuspid valve, regurgitation (backflow of blood) of varying degrees is recorded.
- Cardiac catheterization and right contrast atriography.
Forecast
Early onset of the disease in childhood or adolescence is a prognostically unfavorable sign.
When severe disorders are diagnosed, the probability of survival of a newborn is 75% during the first month of life. 68% survive up to six months, 64% survive up to 5 years, and subsequently the mortality curve stabilizes.
The likelihood of death during surgery depends on the severity of the anomaly in each specific case and the presence of concomitant pathology. 90% of patients after surgical treatment have positive immediate and long-term results. Restoration of working capacity is possible within a year.
Signs and symptoms
Let's look at the symptoms of infectious mononucleosis, which is a manifestation of a child's primary contact with the Epstein-Barr virus. Sometimes mononucleosis in children is caused by cytomegalovirus (CMV; differential serological testing is always necessary). The disease begins acutely and lasts from 3 to 4 weeks.
With mononucleosis (if it is caused by EBV and not cytomegalovirus), the following symptoms appear. It is discovered during direct examination of the child. :
1. Increase in body temperature to 39-40 degrees with severe intoxication syndrome – nausea, vomiting, weakness, headache, tachycardia;
2. Enlarged lymph nodes throughout the body (especially in the neck - anterior and posterior cervical nodes);
3. Nasopharyngitis and tonsillitis with white-gray or yellowish plaques (due to damage to the tonsils and adenoids);
4. Difficulty nasal breathing in the absence of discharge from the nasal passages, puffiness of the face, nasal voice;
5. Enlarged liver and spleen (hepatosplenomegaly in children), pain in the abdominal cavity, icterus of the sclera and skin;
6. Exanthema (rash of viral origin) in the form of spots, papules, vesicles with widespread localization.
Analyzes:
During a microscopic examination during the period of acute infection, among ordinary blood cells, large atypical lymphocytes are found that are affected by the virus - mononuclear cells (this picture of blood is sometimes given by cytomegalovirus, CMV). They remain in the bloodstream for a month from the moment of infection.
For serological diagnosis of mononucleosis, enzyme-linked immunosorbent assay (ELISA) or polymerase chain reaction (PCR) is used, which detects the Epstein-Barr virus.
What antibodies (AT) of the IgG and IgM types (immunoglobulin M, G) are diagnostic when performing an IF analysis?
| Type of antibodies | Characteristic |
| Anti-VCA IgM antibodies (immunoglobulin M to capsid antigen) | Produced during acute EBV infection, they circulate in the blood for 2-3 months. They are resynthesized in case of virus reactivation. A high concentration of anti-VCA IgM, which persists for a long time, is evidence of a chronic form of EBV. |
| Anti-EA IgG antibodies (immunoglobulin G to early antigen) | They appear in the blood 3-4 weeks after the onset of acute EBV infection and persist for 2-6 months. Anti-EA IgG reappears when the pathogen is reactivated. |
| Anti-EBNA IgG antibodies (immunoglobulin G to nuclear antigen) | They begin to circulate in the bloodstream 1-6 months after the primary EBV disease. Gradually their concentration decreases. Anti-EBNA IgG can be detected until the end of a person's life (they are always detected by IF analysis). |
If an IF analysis was performed, a positive result revealed
:
· IgG antibodies (immunoglobulin G) against nuclear and early antigens;
IgM antibodies (immunoglobulin M) to the capsid (VCA) antigen of the virus