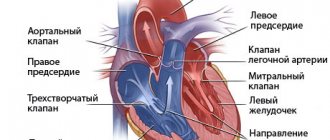
Cardiac output is a concept used in medicine that refers to the amount of blood ejected by the heart in one minute. Technically, it is calculated as the product of heart rate and stroke volume.
An increase in heart rate is a compensatory mechanism for increasing oxygen supply to tissues.
Factors that influence systolic volume are the volume of blood pushed by the heart into the aorta during the contraction period, preload, afterload, and contractile function.
Today, there are a number of techniques that allow us to obtain cardiac output measurements in a very efficient and non-invasive way. The Fick technique, which was used previously, has been replaced by more modern methods.
Pulse wave analysis resulted in a continuous and minimally invasive measurement of cardiac output. Other techniques, such as bioremediation, Doppler or echocardiography, allow us to obtain cardiac output measurements in a non-invasive, rapid and reliable manner.
Strengthening the heart muscle
Digitalis is one of the oldest drugs still used, mainly as a derivative known as digoxin (Lanoxin). The muscles of the ventricles contract to remove blood from the heart. Digoxin increases the strength and speed of this contraction. It may also help control abnormal heart rhythms that reduce EF. Other drugs similarly increase the pumping effect, such as milrinone (Primacor) and dobutamine (Dobutrex). These drugs are not generally recommended for heart failure and are reserved for the closely monitored treatment of acute heart failure that is refractory to other treatments.
Talk to your healthcare provider about improving your EF.
Your doctor can help you come up with a realistic plan. What works for another person may not work for you. Ask your doctor or other health care provider about his or her recommendations, and accept the support and encouragement of family members or friends who can help you achieve your goals.
Before deciding on next steps, first discuss the following questions with your doctor:
- What is my EF?
- Why do I have low EF and how does it affect my health?
- What lifestyle changes or medications might help me? Is there anything I should avoid? Treat any known causes of heart failure.
Find out if your heart failure is due to an underlying cause. There are several risk factors associated with heart failure, such as high blood pressure or hypertension, diabetes, and metabolic syndrome. By managing these conditions, you can help heart failure and ejection fraction improve.
Know your plan and follow it carefully. If you have been prescribed medications for heart failure, diabetes, high blood pressure, or another underlying cause, taking your prescribed medications may also improve your ejection fraction. Over time, as the medications work, your heart can recover, become stronger, and function better.
Relevance of the problem
Over the past 20 years, the incidence of heart failure among Europeans has been decreasing. But the number of cases in the middle and older groups of the population is increasing due to increasing life expectancy.
According to European studies (ECHOCG), a decrease in ejection fraction was found in half of patients with symptomatic heart failure and in half of asymptomatic patients.
Patients with heart failure are less able to work, their quality of life and its duration are reduced.
Treatment of these patients is the most expensive for both them and the state. Therefore, the search for ways to prevent the occurrence, early diagnosis and effective treatment of heart disease remains relevant.
Studies conducted in recent decades have proven the effectiveness of a number of groups of drugs to improve the prognosis and reduce mortality in patients with low cardiac fraction:
- adenosine-converting enzyme inhibitors (Enalapril);
- angiotensin P antagonists (Valsartan);
- beta blockers (“Carvedilol”);
- aldosterone blockers (“Spironolactone”);
- diuretics (“Torasemide”);
- "Digoxin".
Heart failure with restored ejection fraction: diagnostic criteria and treatment strategies
Current recommendations for the diagnosis and treatment of heart failure (HF) distinguish three variants depending on the ejection fraction: HF with reduced ejection fraction (<40%), HF with intermediate ejection fraction (40-49%) and HF with preserved ejection fraction ( ≥50%). However, it is clear that in a significant proportion of patients, the ejection fraction may change as the disease progresses. Moreover, this change often occurs not in the direction of decreasing it, but, on the contrary, in the direction of increasing it. Thus, according to observational studies, from 10 to 40% of patients move from the category of HF with low ejection fraction to the category of HF with intermediate/preserved ejection fraction. Various guidelines provide little discussion of the management of these patients, and the JACC Advisory Panel has prepared a document addressing the issue of HF with reduced ejection fraction.
It is noted that the reason for issuing this document was the almost complete lack of information discussed above about the management of such patients in standard recommendations for HF, as well as an attempt to draw attention to the problem of HF with restored ejection fraction.
Speaking about the diagnosis of heart failure with reduced ejection fraction, experts noted that the working definition should be the presence of three criteria:
1. Information about a decrease in ejection fraction <40% in the anamnesis;
2. Absolute improvement in ejection fraction ≥10%;
3. Re-measurement ejection fraction >40%.
These are the criteria used in the vast majority of studies of heart failure with reduced ejection fraction.
Given the lack of information on the effects of long-term treatment withdrawal in these patients, as well as data from the TRED-HF study, which demonstrated worsening of HF even after recovery of ejection fraction when treatment was discontinued in patients with dilated cardiomyopathy, it is emphasized that all patients with HF and restored ejection fraction should continue taking the therapy indicated in the recommendations for patients with heart failure and low ejection fraction.
Regarding the management of such patients, as the authors note, it should be remembered that despite the restored ejection fraction, such patients have an increased risk of developing cardiovascular complications. In addition, it is possible that the ejection fraction will decrease again. In this connection, it is recommended to perform echocardiography every 6 months, including assessment of left ventricular deformation, every 6-12 months - electrocardiography (ECG), as well as a study of the concentration of brain natriuretic peptides. If there is reason to believe that there is an increased risk of developing cardiac arrhythmias (for example, with transthyretin amyloidosis), Holter ECG monitoring is recommended every 1-2 years. It has been discussed that after a year of clinically stable HF with restored ejection fraction, MRI may be considered if it was not performed during HF with low ejection fraction.
Source:
Wilcox JE, et al. J Am Coll Cardiol. 2021 Aug 11;76(6):719-734.
Reduced vascular resistance of a blood vessel
Arteries also have muscles. The more they contract, the smaller the channel through which blood flows, and the greater the tension on the heart muscle. Angiotensin, a naturally occurring hormone, is a very powerful blood vessel constrictor. Drugs that inhibit the formation of angiotensin or block its ability to bind to a blood vessel help relax the vascular muscles and are the basis of the Heart Failure Society of America Guidelines. Examples include captopril (Capoten), enalapril (Vasotec), valsartan (Diovan), and losartan (Cozaar). Because of this relaxation, the heart can pump out more blood per beat, improving EF. The drug combination hydralazine and isosorbide (Bidil) may be effective in patients who are resistant to—or intolerant to—angiotensin inhibitors.
What results can I expect?
Although individual results may vary, efforts to improve your heart's ejection fraction may have additional benefits. You may also find that you feel better and experience fewer symptoms. The investments you make to help yourself get better are your best way to take control of your health.
Connect with others in our support network, where people encourage each other to achieve better health every day.
Based on materials from the site: www.heart.org
Relaxation of the heart muscle
In response to decreased EF, the body releases substances such as adrenaline, which increase the speed and force of ventricular contraction. While temporary effective, chronic elevations of these compounds cause the heart muscle to strengthen and reduce its movement. Beta blockers are drugs that counteract these actions, reducing tension in the heart wall and improving the ability to contract. Three of these are recommended: carvedilol (Coreg), metoprolol succinate (Toprol XL), and bisoprolol (Zebeta). Beta blockers are most often used in combination with angiotensin inhibitors.
Muscle pump
The movement of blood through the veins is ensured by a number of factors: the work of the heart, the valve apparatus of the veins, the “muscle pump”, etc. The veins of the upper and lower extremities are equipped with valves, and the deep veins are surrounded by muscles. With physical
When under load, the muscles act like pumps, putting pressure on the veins from the outside. The more frequent and active the movements, for example when walking, the more effective the “pumping action” of the muscles. True, muscle contraction, pinching blood vessels, impedes blood flow. But if the contractions are intermittent, then the decrease in blood flow during the contraction phase is effectively compensated by oxygen bound to myoglobin. Therefore, during rhythmic exercise that occurs when running, skiing, or cycling, the blood supply to the muscles of the limbs increases significantly. Contraction of the abdominal muscles forces a significant amount of blood out of the vessels of the liver, intestines and spleen, increasing blood flow to the heart and thereby affecting cardiac output.
When muscles contract, the veins in them are compressed, which immediately leads to an increase in blood flow to the right ventricle (muscle pump). An increase in the outflow of venous blood from the muscles of the lower extremities promotes rapid filling of the heart and, in addition, increases the perfusion pressure in the lower extremities by reducing the pressure in the veins of the leg and foot.
Activation of the muscle pump is accompanied by changes in the post-capillary vessels (mainly veins) of the systemic circulation.
Physical exercise causes a reflex increase in the tension of the walls of the venous vessels in both working and non-working limbs. This tension is maintained throughout the load and is proportional to its severity.
Patients with a functionally single ventricle of the heart after the Fontan procedure. Lecture for doctors
Lecture for doctors “Patients with a functionally single ventricle of the heart after the Fontan operation.” A lecture for doctors is given by pediatric cardiologist Frank Cetta, Jr., MD (USA)
Additional material
Fontan operation: performance criteria, indications and contraindications, risk factors
M.V. Sprindzhuk, junior researcher, Republican Scientific and Practical Institute, Minsk, Belarus
The Fontan operation has been used for palliative surgical treatment of cyanotic defects since 1971. The fundamental basis for the development of this surgical intervention was laid in the early 40s. XX century
Rice. 1. Performing an anastomosis between the superior vena cava and the right pulmonary artery
Rice. 2. Fontan operation performed with an extracardiac conduit; 1 - right pulmonary artery; 2 - aorta; 3 - superior vena cava; 4 - conduit; 5 - pulmonary trunk stump; 6 - inferior vena cava
Fontan's principles of circulation. To imagine the Fontan circulation, the right ventricle (in the case of corrected tricuspid atresia), which normally pumps blood to the lungs, must be excluded from the normal circulation. The uniqueness of the operation is that hemodynamic conditions are created for the effective functioning of the pulmonary circulation in the presence of a corrected congenital anatomical defect.
The energy to propel blood into the lungs in the Fontan circulation is primarily the result of the pumping function of the left ventricle and the contraction of the right atrium. The blood, having received a push from the ventricle, passes through the systemic vessels, returns to the right atrium and flows into the lungs. Previously, it was believed that for a successful result of the Fontan operation, a sufficiently developed chamber of the right atrium was necessary, which is mainly found in relatively adult patients. In fact, prolonged overload of this chamber leads to an increase in its capacity, hypertrophy of the walls and, as experience shows, in this case the right atrium successfully performs the function of the right ventricle. However, atrial expansion contributes to the development of arrhythmias and thrombosis with subsequent embolism.
It is known that vascular resistance slows and ultimately stops the flow of fluid, which is why the hemodynamic characteristics of the patient must be taken into account for a successful surgical outcome. For example, if the pulmonary vessels are thick-walled, with a narrow lumen, they will provide significant resistance to pulmonary blood flow, which will not be possible without additional force.
Conditions for the effective implementation of the Fontan operation. There are several options for criteria for the Fontan operation. The most used criteria are S. van Doorn and MR de Leval, subsequently refined [10]:
| Original criteria | Revised version |
| 1. Minimum age - 4 years | Minimum age is less than 4 years. The minimum and optimal age for performing the Fontan procedure is unknown. |
| 2. Sinus rhythm | Sinus rhythm is desirable |
| 3. Normal drainage of the vena cava | Normal drainage of the vena cava is desirable |
| 4. Right atrium of normal volume | A small right atrium is a contraindication for lateral Fontan tunnel surgery. |
| 5. Average pulmonary artery pressure is less than 15 mm Hg. Art. | The average pulmonary artery pressure is less than 15 mm Hg. Art. |
| 6. Pulmonary alveolar resistance - less than 4 units/m2 | Pulmonary alveolar resistance - less than 4 units/m2 |
| 7. The ratio of the diameters of the pulmonary artery and aorta is more than 0.75 | Adequate dimensions of the pulmonary vascular bed, the use of the McCoon and Nacato indices may be useful |
| 8. Normal ventricular function (ejection fraction more than 0.6) | Preserved ventricular systolic and diastolic function |
| 9. Competent left atrioventricular valve | Competent left atrioventricular valve |
| 10. No disturbing effects of previous shunts | No disruptive effects of previous shunts |
The criteria of A. A. Choussat (1977) are also widespread, although they differ little from the previous ones [9]:
1) age limits - more than 4 and less than 15 years;
2) normal sinus rhythm;
3) normal venous return;
4) normal volume of the right atrium;
5) average pulmonary artery pressure - less than 15 mm Hg. Art.;
6) pulmonary arteriolar resistance - less than 4 units. Wood per 1 m2 of body surface area;
7) ratio of the diameters of the pulmonary artery and aorta - more than 0.75;
 left ventricular ejection fraction - more than 0.60;
left ventricular ejection fraction - more than 0.60;
9) competent mitral valve;
10) absence of distortion of the pulmonary arteries.
Indications and contraindications for the Fontan procedure
This operation was originally proposed for use in cases of absence of the right atrioventricular junction; it is now being used in connection with the expansion of indications for surgical treatment of many complex cardiac malformations with only one well-developed ventricle. Such defects include right or left atrioventricular valvular atresia, double inflow ventricle, pulmonary atresia with an intact ventricular septum, hypoplastic right or left ventricle with ventricular septal defects, with or without the presence of an overlying atrioventricular valve, and isolated cases of double origin of the great vessels from the same ventricle.
Performing the Fontan procedure has only a few absolute contraindications:
pulmonary vascular resistance - more than 4 units. Wood per 1 m2;
severe hypoplasia of the pulmonary arteries;
severe left ventricular diastolic dysfunction.
The age at which surgery is performed has dropped significantly, with some clinical centers performing the procedure as early as 2 years of age or even earlier in patients with suitable anatomy and physiology. The benefit of performing the Fontan procedure at an early age is increased oxygenation, which improves somatic growth and neurodevelopmental outcomes. The operation also reduces the volume load on the single ventricle.
It is believed that the operation should be performed after the child begins to walk or at least crawl, in order to take advantage of the effect of muscle contraction on venous return. The presence of preoperative sinus rhythm is not a prerequisite for a successful outcome of the operation, but it is necessary to ensure tempo in the immediate postoperative period. Abnormal venous return is not an absolute contraindication for surgery and is considered compatible with the Fontan circulation. Assessing ventricular function is difficult due to the heterogeneous geometry of many single ventricles. Magnetic resonance imaging allows accurate measurement of ventricular ejection volumes and regurgitant fractions, but it is unclear whether these volumetric measurements are useful as predictors of outcome after the Fontan procedure.
The presence of atrioventricular valve regurgitation may be a consequence of volume overload of a single ventricle if the patient has a systemic-pulmonary shunt; regurgitation may improve when the volume load is removed.
There are reports of good tolerability of atrioventricular valve regurgitation after the Fontan procedure. However, current management involves aggressive attempts to correct or reduce atrioventricular valve regurgitation as much as possible [7, 10–14].
In a study by S. Ovrutski et al. [15] end-diastolic systemic ventricular pressure (ESVDP) more than 10 mm Hg. Art. and mean pulmonary artery pressure (MPAP) above 15 mm Hg. Art. are risk factors. However, the authors believe that a CDVD of 12 mmHg. Art., and MPAP equal to 17 mm Hg. Art., are acceptable indicators for performing the Fontan procedure with adequate ventricular function.
Types of operation. Of course, the feasibility, type of operation and time of its implementation must be determined for each case individually, taking into account the facts that there are no two identical hearts and the anatomy of the conduction system and each patient has unique hemodynamic parameters, the ability to adapt to pathology and reserve strength to endure the operation.
Analysis of literature sources allows us to draw several conclusions regarding the choice of the type of operations to correct single ventricles of various shapes. First, for an unbalanced (asymmetrical) atrioventricular canal, the procedure of choice is biventricular repair, or the so-called sesquiventricular modification of the Fontan procedure using a bidirectional Glenn anastomosis to reduce the amount of blood passing through the relatively small right ventricle and tricuspid valve (in other words, “unload " his). Single-ventricular correction is only necessary in rare cases when there is significant right ventricular outflow obstruction or a small ventricular component, as well as in patients with significant imbalance (asymmetry) of the common AV limiting right ventricular flow, to restrict blood flow to the right ventricle. The hypothetical benefits of sesquiventricular repair for an unbalanced (asymmetric) atrioventricular valve are:
1) the ability to avoid high system pressures;
2) antegrade, pulsatile blood flow directed to the pulmonary arteries initiates potential growth of the pulmonary arteries;
3) reduced risk of long-term complications of Fontan circulatory physiology - exercise intolerance, atrial arrhythmias and protein-deficiency enteropathy [16-18].
A diagnosis of tricuspid atresia (as well as mitral atresia and hypoplastic left heart syndrome) is a clear indication for cavapulmonary anastomosis, and a history of previous surgery is associated with higher rates of complications. Even performing modifications of the Fontan procedure with the creation of a complete cavapulmonary anastomosis after superior cavapulmonary anastomosis operations (hemi-Fontan and bidirectional Glenn shunt) can increase the incidence of problems such as arrhythmias, thromboembolism, pleural extravasation, protein-deficient enteropathy and obstructive bronchitis, central and peripheral neurological complications.
Yueh-Tze Lan et al. [19] reported better long-term results in patients with a single ventricle in the presence of a double inlet (double-inlet ventricle) than in the case of tricuspid atresia with transposition of the great vessels. Late arrhythmias and the need for a pacemaker were identified as independent risk factors for death. Systemic outlet obstruction, when recognized and corrected early, has no impact on long-term survival rates. Vascular protection by naturally occurring pulmonary or subpulmonary stenosis, as well as by pulmonary artery banding, was associated with better survival.
The anatomical diagnosis for which the literature indicates the best natural outcome (without surgical intervention - the Fontan procedure or an alternative intervention - the separation operation [Fig. 3]) is a single ventricle with a double inlet (double-inlet single ventricle), in other words, double-inlet. Obviously, this should be taken into account before referring an adult patient to the Fontan procedure, for which a 10-year survival rate is established in 65-85% of cases, and the risk of postoperative mortality can reach 30% or more.
For clinically severe Ebstein's anomaly, an option for single-ventricle correction (as for an asymmetric atrioventricular valve) is sesquiventricular correction. In this pathology, it is believed that the main advantages of including a hypoplastic (pulmonary) ventricle to partially support the pulmonary circulation are:
1) the ability to increase cardiac output;
2) adaptation to physical exercise;
3) maintaining pulsatile flow in the pulmonary circulation;
4) flexibility in response to increased pulmonary resistance;
5) circulation at low pressure levels in the inferior vena cava system;
6) the ability of the hypoplastic complex of the right heart to adequately cope with reduced preload
Rice. 3. Septation procedure [20, 21]: 1 — pulmonary trunk; 2 - bulboventricular opening; 3 - tricuspid valve; 4 - patch
JM Stulak et al. [22] report that the one-and-a-half procedure is used in cases where the right ventricle is considered unable to support pulmonary circulation. The researchers believe that sesquiventricular repair may serve as an alternative for patients with severe Ebstein's anomaly and poor right ventricular function, who may be at high risk with standard surgical treatment. In addition to reducing the load, according to the researchers, performing the operation introduces preload to the left ventricle.
In the case of surgical treatment of pulmonary atresia, which occurs as part of complex defects, judging by the literature, it is difficult to unambiguously judge the success of single-ventricle correction. H. Leonard et al. [23] reported on an examination of 57 of 129 births with pulmonary atresia in 1980–1995. The authors concluded that treatment outcomes were disappointing and that patients who underwent biventricular repair (see Figure 3) had better exercise capacity than those who underwent single-ventricular repair. The researchers also reported a high rate of sudden death in patients with pulmonary atresia, which was 29/1000 patient years. However, DD Mair et al. [24], examining 860 Fontan procedures from 1973 to 1994, concluded that patients with pulmonary atresia and an intact ventricular septum were not candidates for conventional biventricular surgery if the tricuspid valve was at least 70% of its expected size. In patients with its size in the range of 50-70%, only biventricular correction with bidirectional Glenn anastomosis can be performed. In patients with less than expected tricuspid and right ventricular dimensions, the Fontan procedure provides “definitive palliation,” and the early and late results of this approach are encouraging.
Extracardiac, extracardiac conduit is a flexible operation in terms of indications and can be performed in almost all anatomical situations. These include cases in which other approaches may be difficult (eg, patients with heterotaxy syndrome and abnormalities of systemic and pulmonary venous drainage).
Compared with previously used techniques of the Fontan procedure, the frequency of pleural transudation after extracardiac bypass operations of the right heart is significantly higher. The reason for this is not completely clear - perhaps this is due to the lack of fenestration, which allows to relieve the pulmonary circulation, which has not fully adapted to the increased blood flow. However, researchers do not consider it necessary to perform fenestration in all patients, since the symptoms of hydrothorax can be managed, and the presence of arterial hypoxemia will not improve the patient’s condition and may require a further fenestration closure procedure. In addition, in this group, such serious complications as acute heart failure and tachyarrhythmias, to avoid which fenestration was performed during the operation of total cavapulmonary anastomosis, practically do not occur [25].
Conclusion. Since the development of the Fontan procedure, indications for its implementation have expanded due to the creation of new modifications and the study of postoperative effects. The ideal Fontan operation has not yet been created, although the modification by C. Marcelletti is close to it. There is no clear opinion, taking into account the individual hemodynamic parameters of the patient and variations in operations, whether it is advisable to perform fenestration in patients undergoing a Fontan procedure with an extracardiac conduit. Based on the literature, intervention as early as possible is preferable.
Analysis of literature data suggests the prospects for further development of the operation of dividing a single ventricle, and also indicates ways to improve other operations.
Literature
1. Bockeria L.A., Podzolkov V.P., Glyantsev S.P., Kokshenev I.V. Operation of cavapulmonary anastomosis. Part 2. From implementation to its improvement. Children's Heart and Vascular Diseases 2005; 5: 7-13.
2. Baum CV Pediatric cardiac surgery: historical appreciation. Pediatric Anesthesia 2006 May: 1-12.
3. Bjork VG Fifty years of cardiac and pulmonary surgery. Scand J Thoracic Surgery Supplement 1998; 8: 142-149.
4. Galankin NK Cavapulmonary anastomosis. M: Medytsina; 1968; 350 p.
5. Vishnevsky AA, Galankin NK, Krymski LD Tetralogy of Fallot. M: Medytsina; 1969: 225 p.
6. Keirle A., Helmsworth JA, Kaplan S., Ogden A. Experience with fnastomosis of superior vena cava to pulmonary artery glenn procedure. Circulation 1963 April; 24: 753–760.
7. Kirklin's J., Barrat-B's. Cardiac surgery. Wiley & Sons; 2005; 2583 p.
8. Robicsek F., Watts LT A prelude to Fontan. Pediatr Car-diol 2007; 28(6): 422–425.
9. Wilkinson JL The Fontan simulation. Results, late follow-up and management. Heart Views 2004; 4(4): 73-78.
10. Van Doorn CA, de Leval MR The lateral tunnel Fontan. Oper Tech in Thorac and Cardiovasc Surg 2006; 11(2): 105–122.
11. Bradley SM Extracardiac Fontan procedure. Oper Tech in Thorac and Cardiovasc Surg 2006; 3(5): 123–140.
12. De Leval MR The Fontan circulation: a challenge to Wil-liam Harvey? Nature Clinical Practice Cardiovascular Med-icine 2005; 2: 202–208.
13. Hosein Riad BM, Andrew JB Clarke, Simon P. McGuirk et al. Factors influencing early and late outcome following the Fontan procedure in the current era. The "Two Commandments"? Eur J Cardiothorac Surg 2007; 31; 344-353.
14. Uemura H, Yagihara T., Kawashima Y, Yamamoto F., Nishigaki K, Matsuki O, Okada K, Kamiya T., Anderson RH What factors affect ventricular performance after a Fontan-type operation? J Thorac Cardiovasc Surg 1995; 110(2): 405–415.
15. Ovrutski S., Alexi-Meskishvili, Ewert P., Nurnberg J.-H., Hetzer R., Lange P.E. Early and medium-term results after modified Fontan operation in adults. Eur J Cardiothorac Surg 2003; 23: 311–316.
16. Cohen MS, Spray TS Surgical management of unbalanced atrioventricular canal defect. Semin Thorac Cardiovasc Surg Pediatr Card Surg Ann 2007; 8: 135–144.
17. Corno AF, Chassot PG, Payot M., Sekarski N., Tozzi P., von Segesser LK Ebstein's anomaly: one and a half ven-tricular repair. Swiss Med Wkly 2002; 132: 485–488.
18. Mitchell ME, Litwin SB, Tweddell JS Complex atrioventricular canal. Semin Thorac Cardiovasc Surg Pediatr Card Surg Ann 2007; 10: 32-41.
19. Yueh-Tze Lan, Ruey-Kang Chang, Hillel Laks, MD. Out¬come of patients with double-inlet left ventricle or tricuspid atresia with transposed great arteries. J Am Coll Cardiol 2004; 43: 113–119.
20. CTS files. Cardiac Surgery files; 2007.
21. https://www.perfusionkorea.org/kosect/chd/singleventricle.php
22. Stulak JM, Dearani JA, Danielson GK Surgical man-agement of Ebstein's anomaly. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 2007; 10: 105–111.
23. Leonard H., Derrick G., O'Sullivan J., Wren C. Natural and unnatural history of pulmonary atresia. Heart 2000; 84(5): 499–503.
24. Mair DD, Puga FJ, Danielson GK The Fontan procedure for tricuspid atresia: early and late results of a 25-year experience with 216 patients. J Am Coll Cardiol 2001; 37(3): 933–939.
25. Podzolkov V.P., Chiaureli M.R., Zelenikin M.M. et al. Immediate and mid-term results of the Fontan operation in modification of the extracardiac conduit. Thoracic and Cardiovascular Surgery 2005; 4: 10-13.