The phenomenon of osmotic pressure in water was discovered and described back in 1748 by the French experimental physicist Jean-Antoine Nollet.
Conducting his experiment, Nolle filled a vessel with ethanol and, closing it with a dense membrane, lowered it into a container of clean water. Under the influence of physical forces, water entered the vessel with concentrated liquid and created pressure there, under the influence of which the vessel swelled. During his experiment, five hours was enough for the volume in the vessel to increase and the membrane to swell. Then he decided to conduct the opposite experiment and filled the flask with water, placing it in a vessel with alcohol. The volume in the flask began to decrease, and the membrane began to sag downward.
Nolle explained this phenomenon as the selective transfer of molecules through a membrane: when a liquid with a lower density easily passed through the walls of the membrane, the second, concentrated one, could not diffuse.
It was later proven that if pressure is applied to a concentrated solution, the transfer of solvent molecules can be slowed down or stopped depending on the amount of pressure. The smallest pressure, excluding the pressure of the solvent itself, that must be applied to the solution in order to prevent the movement of molecules of a pure substance through the membrane was called “somatic”, and the process of random transfer of solvent molecules began to be called “osmosis”.
What does the osmotic pressure of water depend on?
An important condition for osmosis is the presence of a semi-permeable membrane, that is, a material whose pores are of sufficient size to freely pass solvent molecules and retain solute particles in solution.
The osmotic pressure of water depends on two main factors:
- solution concentration;
- temperature.
This is explained by the van't Hoff equation. The osmotic pressure of water is: π = RCT,
where R is the universal gas constant,
C is the concentration of the substance,
T is temperature.
The scientist discovered that the osmotic pressure of liquid solutions obeys the same laws as the pressure of gas systems. Using this equation, the pressure value is determined.
It does not depend on the composition of the dissolved substance, therefore osmotic pressure is considered a colligative property of a solution, that is, due to the spontaneous movement of molecules, their number, and not composition.
For the occurrence of osmotic pressure of water in the system, two criteria are necessary:
- the presence of a semipermeable membrane;
- finding two solutions with different concentrations on both sides of the partition.
What it is?
Osmotic pressure is the value at which the process of osmosis is completed .
Osmosis, in turn, is the transfer of molecules of substances from one solution to another. They usually move from a less concentrated, low-content substance to a more concentrated one. Movement occurs through a thin wall - a membrane.
Simple example:
Take a vessel that has thin walls, tapering upward to a tube of small diameter.- Fill it with a mixture of water and sugar.
- Place in another container filled with water.
- There will be a gradual increase in the mixture, filling the vertical tube. This is due to the phenomenon of osmosis: particles begin to “run across” a thin wall.
- The higher the mixture rises through the vertical tube, the greater the pressure will be created.
When it reaches a certain point, the osmosis process will stop. This is osmotic pressure.
How to determine the osmotic pressure of water
In practice, the osmotic pressure of water is determined using a special device - an osmometer. So measurements can take place statically and dynamically.
With the static method, measurement is carried out only after equilibrium has been established in the system: solution - membrane - solvent. In the simplest way, the value is determined by the height of the liquid column in the osmometer tube. Its disadvantages include the difficulty of determining the moment of equilibrium and significant time costs.
The dynamic method for determining the osmotic pressure of water allows you to quickly and accurately obtain results. It is based on determining the volumetric rate of transmission and extrusion of solvent molecules through membranes with different pressures in the cell, followed by calculation of intermediate values among the results obtained.
Many instruments allow calculations using both methods. The only important condition for carrying out the measurement is the correct selection of the semi-permeable membrane. In practice, the following are most often used:
- cellophane films;
- natural and synthetic polymers;
- porous ceramic and glass partitions;
- membranes of plant and animal origin.
How and when was it opened?
This phenomenon was first discovered and described in 1748. This was done by the French physicist Jean-Antoine Nollet.
His experiment looked like this:
- The container was filled with ethanol and covered with a thin elastic film.
- The vessel was lowered into another, previously filled with water.
- After some time, the thin film begins to swell and inflate. This means that the process of transfer of molecules from one vessel to another has begun.
- They try to swap the vessels: place water in ethanol. The picture is completely opposite: a thin film begins to fall inward.
Jean-Antoine Nollet explained this phenomenon as follows: water, which has a lower density, passes through the film without the slightest resistance. But the higher the concentration, the more difficult it is for molecules to “run across” the barrier.
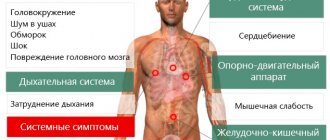
The role of osmotic pressure of water for living organisms
Osmosis is of great importance in the environment and human activities. For example, it is involved in the transfer of fluid in the trunks of tall trees, in the filling of cells and intercellular structures of living organisms with water. Human biological fluids - tissue fluids, blood, lymph are also subject to the laws of osmotic pressure. In laboratory conditions, it is used to study the characteristics of newly obtained polymer substances, and in industry it is used to purify water from minerals using reverse osmosis.
Osmosis plays an equally important role in the ecology of water bodies. When the concentration of salts in water changes, the inhabitants may die, for example, if a freshwater animal is placed in sea water, it will soon lose a fifth of its weight, and if a sea animal is transferred to fresh water, then due to the diffusion of molecules the level of intracellular fluids, the cells of his organs will swell and burst.
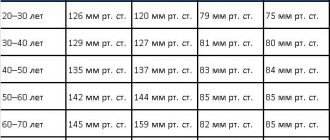
Water-electrolyte metabolism in the body of a healthy person: principles of regulation
Regulation of water-salt metabolism, like most physiological regulations, includes afferent, central and efferent links. The afferent link is represented by a mass of receptor apparatus of the vascular bed, tissues and organs that perceive shifts in osmotic pressure, volume of liquids and their ionic composition. As a result, an integrated picture of the state of water-salt balance in the body is created in the central nervous system. Thus, with an increase in the concentration of electrolytes and a decrease in the volume of circulating fluid (hypovolemia), a feeling of thirst appears, and with an increase in the volume of circulating fluid (hypervolemia), it decreases. The consequence of the central analysis is a change in drinking and eating behavior, a restructuring of the gastrointestinal tract and excretory system (primarily kidney function), implemented through efferent regulatory links. The latter are represented by nervous and, to a greater extent, hormonal influences. An increase in the volume of circulating fluid due to increased water content in the blood (hydremia) can be compensatory, occurring, for example, after massive blood loss. Hydremia with autohemodilution is one of the mechanisms for restoring the correspondence of the volume of circulating fluid to the capacity of the vascular bed. Pathological hydremia is a consequence of impaired water-salt metabolism, for example, in renal failure, etc. A healthy person may develop short-term physiological hydremia after taking large amounts of liquid.
In addition to the permanent exchange of water between the body and the environment, the exchange of water between the intracellular, extracellular sectors and blood plasma is important. It should be noted that the mechanisms of water-electrolyte exchange between sectors cannot be reduced only to physicochemical processes, since the distribution of water and electrolytes is also related to the functioning of cell membranes. The most dynamic is the interstitial sector, which primarily affects the loss, accumulation and redistribution of water and shifts in electrolyte balance. Important factors influencing the distribution of water between the vascular and interstitial sectors are the degree of permeability of the vascular wall, as well as the ratio and interaction of hydrodynamic pressures of the sectors. In plasma, the protein content is 65-80 g/l, and in the interstitial sector only 4 g/l. This creates a constant difference in colloid-osmotic pressure between the sectors, ensuring the retention of water in the vascular bed. The role of hydrodynamic and oncotic factors in the exchange of water between sectors was shown back in 1896. American physiologist E. Starling: the transition of the liquid part of the blood into the interstitial space and back is due to the fact that in the arterial capillary bed the effective hydrostatic pressure is higher than the effective oncotic pressure, and in the venous capillary - vice versa.
Humoral regulation of water and electrolyte balance in the body is carried out by the following hormones:
- antidiuretic hormone (ADH, vasopressin), acts on the collecting ducts and distal tubules of the kidneys, increasing water reabsorption; - natriuretic hormone (atrial natriuretic factor, ANF, atriopeptin), dilates afferent arterioles in the kidneys, which increases renal blood flow, filtration rate and Na+ excretion; inhibits the release of renin, aldosterone and ADH; - the renin-angiotensin-aldosterone system stimulates the reabsorption of Na+ in the kidneys, which causes NaCl retention in the body and increases the osmotic pressure of the plasma, which determines the delay in fluid excretion.
— parathyroid hormone increases the absorption of potassium by the kidneys and intestines and the excretion of phosphate and increases the reabsorption of calcium.
The sodium content in the body is regulated mainly by the kidneys under the control of the central nervous system through specific natrioreceptors. responding to changes in sodium content in body fluids, as well as volume receptors and osmoreceptors, responding to changes in the volume of circulating fluid and osmotic pressure of extracellular fluid, respectively. The sodium content in the body is controlled by the renin-angiotensin system, aldosterone, and natriuretic factors. With a decrease in water content in the body and an increase in the osmotic pressure of the blood, the secretion of vasopressin (antidiuretic hormone) increases, which causes an increase in the reabsorption of water in the renal tubules. An increase in sodium retention by the kidneys is caused by aldosterone, and an increase in sodium excretion is caused by natriuretic hormones, or natriuretic factors (atriopeptides, prostaglandins, ouabain-like substance).
The state of water-salt metabolism largely determines the content of Cl- ions in the extracellular fluid. Chlorine ions are excreted from the body mainly through urine, gastric juice, and sweat. The amount of excreted sodium chloride depends on the diet, active sodium reabsorption, the state of the renal tubular apparatus, and the acid-base state. The exchange of chlorine in the body is passively associated with the exchange of sodium and is regulated by the same neurohumoral factors. The exchange of chlorides is closely related to the exchange of water: a decrease in edema, resorption of transudate, repeated vomiting, increased sweating, etc. are accompanied by an increase in the excretion of chlorine ions from the body.
The balance of potassium in the body is maintained in two ways: by changing the distribution of potassium between intra- and extracellular compartments, and by regulating renal and extrarenal excretion of potassium ions. The distribution of intracellular potassium in relation to extracellular potassium is maintained primarily by Na-K-ATPase, which is a structural component of the membranes of all cells of the body. The absorption of potassium by cells against the concentration gradient is initiated by insulin, catecholamines, and aldosterone. It is known that acidosis promotes the release of potassium from cells, while alkalosis promotes the movement of potassium into cells.
The fraction of potassium excreted by the kidneys usually accounts for approximately 10-15% of the total filterable plasma potassium. The retention in the body or the excretion of potassium by the kidney is determined by the direction of potassium transport in the connecting tubule and collecting duct of the renal cortex. When the content of potassium in food is high, these structures secrete it, but when it is low, there is no secretion of potassium. In addition to the kidneys, potassium is excreted through the gastrointestinal tract and through sweating. At the usual level of daily potassium intake (50-100 mmol/day), approximately 10% is excreted in the stool.
The main regulators of calcium and phosphorus metabolism in the body are vitamin D, parathyroid hormone and calcitonin. Vitamin D (as a result of transformations in the liver, vitamin D3 is formed, in the kidneys - calcitriol) increases the absorption of calcium in the digestive tract and the transport of calcium and phosphorus to the bones. Parathyroid hormone is released when the level of calcium in the blood serum decreases, but a high level of calcium inhibits the formation of parathyroid hormone. Parathyroid hormone helps increase calcium levels and decrease phosphorus concentrations in the blood serum. Calcium is resorbed from the bones, its absorption in the digestive tract also increases, and phosphorus is removed from the body in the urine. Parathyroid hormone is also necessary for the formation of the active form of vitamin D in the kidneys. An increase in serum calcium levels promotes the production of calcitonin. In contrast to parathyroid hormone, it causes the accumulation of calcium in the bones and reduces its level in the blood serum, reducing the formation of the active form of vitamin D in the kidneys. Increases the excretion of phosphorus in the urine and reduces its level in the blood serum.
Potatoes in sugar solution
Type: Exosmos
If you put potatoes in a sugar solution, they will shrink over time. This is because the potato cells have a much higher concentration of water than the sugar solution, so the water leaves the potato through its membrane into the sugar solution.
As the solvent leaves the potato cells and enters the sugar solution in an attempt to reach equilibrium, this is an example of exosmosis.
Digested food is absorbed in the small and large intestines
Type: Endosmos
When you drink water or eat food, it moves from your mouth through your esophagus to your stomach. Inside the stomach, food breaks down into many small pieces that mix with stomach fluids. The mixture forms a thick semi-liquid mass called chyme. When chyme enters the small intestine, osmosis occurs.
Intestinal epithelial cells (which form the intestinal lining) have a lower concentration than chyme. Thus, to achieve equilibrium, a solvent (water) enters these cells through semi-permeable membranes, taking some nutrients with it.
Next to the epithelial cells are capillaries. Both nutrients and water pass through the capillary cells into the bloodstream.
Dry eyes caused by contact lenses
Type: Exosmos
Do you know why we put contact lenses in saline solution? Why not clean water? This is because saline contact lens solution contains the same concentration of salt water as your eye.
When you keep your lenses in the solution, they stay moist, soft and comfortable. Otherwise, they tend to absorb moisture from the eyes through osmosis as they lose water during wear.
Pathogenic bacteria interfere with intestinal cells
Cholera bacteria imaged using a scanning electron microscope
Type: Exosmos
Some pathogenic bacteria, such as Vibrio cholerae, are capable of interfering with the ion transport channels of human intestinal cells. They produce enterotoxins, which alter the permeability of epithelial cells of the intestinal wall through the formation of pores.
Osmosis removes water and other fluid compounds from the body, leading to severe dehydration and diarrhea. These bacteria can hold tightly to the intestinal cells while the normal bacteria living in our stomach are washed away. This gives the cholera bacteria enough area to grow and reproduce. Smart little microbe, isn't it?
Application of knowledge for reverse osmosis in practice
The provisions described above formed the basis of another phenomenon - reverse osmosis : a movable partition, like a sieve, allows molecules of a certain size to pass through.
It turns out that solutions have a certain flow through the partition, but large particles do not.
This phenomenon has found application in various fields:
- for filtering water, obtaining fresh water from salt water;
- for the production of various liquids used in production and industry;
- for cleaning water bodies.
Meaning
Osmotic pressure plays an important role in the natural environment and human life:
- Delivers moisture along plant trunks.
- Fills human cells with water. As you know, the body largely consists of it.
- Carries out the movement of various fluids throughout the body.
- It is used in various productions and industries.
- Solutions created based on this method are used in medicine. They are administered intravenously during the rehabilitation period of patients after surgery, as well as for disinfection of wounds and decontamination.
Plants absorb water from the soil
Type: Endosmos
While plants absorb water throughout their entire surface (leaves, stems and roots), most of the water is absorbed by the root hairs. These root hairs act as a semi-permeable barrier, allowing water molecules (solvent) to move from high concentration (soil) to low concentration (roots).
As a result, root hair cells become more swollen, and their osmotic pressure (ability to absorb solvents) drops.
Water molecules then move into tubes called xylem vessels and are transported to the leaves. Within xylem cells, water molecules exert a strong influence on each other through hydrogen bonds. As water evaporates through the stomata (tiny pores on leaves), more water is released through the xylem cells of the root to replace what was lost.
Gargling with salt water relieves sore throat
Type: exosmosis (excess fluid is expelled from the tissues of the throat).
Salt water doesn't actually cure a sore throat, but it does help relieve pain and discomfort. This is because salt water contains a higher concentration of solute (salt) than what is present in the tissues of our throat.
More specifically, the osmotic pressure of salt water is greater than the fluid pressure of the surrounding cells. Therefore, when we gargle, excess fluid is released from the tissues of the throat, reducing swelling and relieving pain.
Food canning
Type: Exosmosis (bacterial cells lose water)
The reason we can enjoy jams and pickles for a long time without fear of spoilage is osmosis. Both are concentrated foods containing large amounts of sugar (in the case of jams), salts, oils, vinegar and other spices (in the case of pickles).
They act not only as flavor enhancers, but also as excellent preservatives, killing bacteria and preventing the growth of other harmful microorganisms.
High concentrations of sugar and salt have a hypertonic effect on bacterial cells. Bacterial cells lose water due to higher concentrations outside and become less conductive to support microbial growth.