General information
Normal lactate levels are low. It increases with a lack of oxygen and disruption of the energy production mechanism. The main amount of energy is produced in mitochondria. This process uses oxygen and glucose. This generation of energy is called aerobic. If this process is disrupted (for example, there is not enough oxygen), energy production occurs anaerobically due to the breakdown of glucose. This produces lactate. If the production of lactic acid exceeds the liver's ability to utilize it, this substance accumulates. In this case, the development of lactic acidosis is possible. In many cases, the body’s compensatory capabilities are sufficient to avoid severe consequences. Sometimes lactic acidosis is not compensated for. In this case, the acid-base balance is disturbed, nausea, weakness, sweating, rapid breathing appear, and coma is possible.
The causes of excess lactate are divided into two groups. The most common is lactic acidosis A. It occurs when blood circulation is slow or oxygen is not being taken up sufficiently by the lungs. This condition can develop with a heart attack, shock from severe blood loss, sepsis, pulmonary edema and in a number of other cases. With type B lactic acidosis, metabolism is impaired and the need for oxygen is increased. It may be caused by kidney or liver disease, leukemia and other diseases.
BLOOD LACTATE LEVEL AS AN INDICATOR OF STAT ANALYSIS
Home \ Publications \ BLOOD LACTATE LEVEL AS AN INDICATOR OF STAT ANALYSIS
BLOOD LACTATE LEVEL AS AN INDICATOR OF STAT ANALYSIS V.A. Torshin, Ph.D., Moscow
The biochemical and clinical interpretation of blood lactate levels appears quite simple at first glance: only one reaction occurs when pyruvate is converted to lactate and vice versa [1]. However, the discovery of understanding the production of lactate from glucose by glycolysis, as well as the conversion of lactate to glucose by gluconeogenesis, is one of the greatest achievements of biochemistry in the 20th century. The energy required for normal functioning and maintenance of cell homeostasis is in the form of adenosine triphosphate (ATP), formed during the oxidation of glucose. The complete oxidation of 1 mole of glucose produces 36 moles of ATP. The aerobic pathway for glucose conversion occurs in two stages. The 1st stage is a chain of sequential reactions, as a result of which in the cytosol of the cell glucose is converted into pyruvate with the formation of 2 moles of ATP. The 2nd stage occurs inside the mitochondrion and includes the Krebs cycle (tricarboxylic acid cycle), while pyruvate is completely metabolized to CO2 and H2O. At the same time, oxidative phosphorylation occurs, resulting in the formation of another 34 moles of ATP. Oxygen is required for the intramitochondrial part of the process to function. Therefore, under anaerobic conditions, pyruvate accumulates in cells. Excess pyruvate is converted to lactate, which diffuses from the cells into the bloodstream. In addition, glycolysis is accompanied by the production of hydrogen ions, which leads to acidosis, first intracellular, then tissue and finally systemic.
The lactate concentration in the extracellular fluid is about 1 mmol/L, reflecting the dynamic process of formation and consumption of 1200 to 1500 mmol lactate during the day [1]. Lactate, as a product of anaerobic glycolysis, is formed in tissues such as skeletal muscles, intestines, brain, skin and red blood cells. Intense exercise can lead to an increase in lactate production in muscles 10 times compared to the resting state. Resynthesis of glucose from lactate occurs in the liver through gluconeogenesis. The processes of gluconeogenesis and oxidative phosphorylation, respectively, consume from 1200 to 1500 mmol of hydrogen per day. That is, the liver plays a more significant role in maintaining the acid-base balance compared to the kidneys, which normally excrete only 60-75 mmol of hydrogen per day.
The ability to determine the level of lactate in the blood of mammals was first demonstrated by Gaglio in 1886. The determination took several days and required about 200 ml of animal blood. In 1940, Barker and Summerson significantly simplified the technique, which allowed Broder and Weil to conclude in 1964 that there was a correlation between blood lactate levels and the severity of shock. Further development led to the creation of even more simplified methods, which were nevertheless recognized as reference.
The importance of blood lactate level in the assessment of patients in critical condition required the use of the method in the STAT analysis mode from a small sample of whole blood in combination with indicators of CBS, electrolyte balance and indicators of oxygen status. These problems became solvable with the development of the amperometric, enzymatic method using a substrate-specific electrode. In work [3], using large clinical material (1218 intensive care patients), a high degree of correlation was proven between reference methods (determining lactate in plasma and whole blood) and a new method that allows monitoring blood lactate levels in patients in critical condition.
The works of [1,2,3,4] and other authors have proven the role of assessing blood lactate levels in patients in critical condition as:
- tissue oxygen debt indicator
- indicator of the effectiveness of therapy
- a prognostic sign of an unfavorable outcome.
The role of lactate as an indicator of tissue oxygen debt has been proven in [3]:
- intense exercise
- circulatory shock (hemorrhagic, cardiogenic, septic)
- cardiac arrest
- severe hypoxemia
- severe anemia
- grand mal seizures
- status asthmaticus
- carbon monoxide poisoning
- sepsis
- vitamin B1 deficiency
- certain types of tumors
- a number of liver diseases
- congenital metabolic disorders
- poisoning with a number of substances (ethanol, methanol, metformin, ethylene glycol, etc.).
The most interesting is the accumulating data on the importance of lactate as a prognostic sign of an unfavorable outcome. A number of authors have proven [1,2,3], firstly, an earlier increase in lactate levels compared to other indicators of developing shock (hypotension, oliguria, decreased pH, etc.). Secondly, there was a clear correlation between blood lactate levels in critically ill patients and mortality rates. The work [4] noted that when lactate increased to 2.7 mmol/l, mortality did not increase, when the level reached 4.0 mmol/l it reached 50%, and when it increased to 8 mmol/l it was 90%. The prognostic role of lactate in newborns is likely to be associated with other reference values. In the group of surviving newborns born with asphyxia, lactate levels of 17-18 mmol/l were recorded [1].
Considering the growing interest among clinicians in such an indicator as the level of blood lactate, we can expect many center studies that can put an end to such problems as determining reference limits, metabolic features of newborns, etc.
Bibliography:
- S.B. Chelnokov, N.A. Pudina
Blood lactate level in newborns born with asphyxia. Materials of the 1st Russian Congress on Pediatric Anesthesiology and Reanimatology. Moscow. 2001; 321-322.
- James A. Kruse
Understanding Blood Lactate Analysis. The Journal for Respiratory Care Practitioners. 1995; 63-69.
- John Toffaletti
Elevations in blood lactate: Overview of use in critical care. Scand J Clin Lab Invest. 1996; 56, Supp224; 107-110.
- Javier Aduen et al.
The Use and Clinical Importance of a Substrate-Specific Electrode for Rapid Determination of Blood Lactate Concentrations. JAMA, December, 7. 1994- Vol 272.No 21.
- John Toffaletti
Blood Lactate: Biochemistry, Laboratory Methods and Clinical Interpretations. Critical Reviews in Clinical Laboratory Sciences. Vol 28, Issue 4; 1991; 253-268.
News all
07/06/2021 X Baltic Forum "Current problems of anesthesiology and resuscitation" June 30 - July 3, 2021
06/03/2021 The 7th Conference “Laboratory Diagnostics of Emergency Conditions 2021” took place
05/11/2021 Conference “Laboratory diagnostics of emergency conditions 2021” June 3, Novotel, St. Petersburg
01/22/2021 Seminar for managers of veterinary clinics
04/09/2020 Manufacturers and suppliers called for simplification of registration of medical devices
When is a lactate test necessary?
The test results enable the doctor to determine the reasons for the disruption in the supply of oxygen to the organs and systems of the body. A blood lactate test is used to identify lactic acidosis and hypoxia and assess their severity. The lactate level makes it possible to differentiate the types of metabolic acidosis. For newborns, analysis is prescribed in case of asphyxia. In sports medicine, the study is used to monitor the condition of athletes during intense training.
The test is prescribed in the following cases:
- suspected lack of oxygen (symptoms such as rapid breathing, sweating, pale skin, shortness of breath, sudden weakness);
- impaired clarity of consciousness, fainting, fever, severe headaches, symptoms of meningitis;
- suspicion of diabetes, sepsis, heart or kidney pathologies, shock.
general characteristics
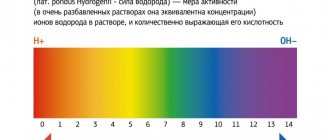
Lactic acid is a product of anaerobic glucose metabolism (glycolysis) and is formed from pyruvate under the action of lactate dehydrogenase when there is insufficient oxygen supply. With sufficient oxygen supply, pyruvate is metabolized in the mitochondria to water and carbon dioxide. The main amount of lactic acid enters the blood from skeletal muscles, brain and red blood cells. Lactate accumulation reduces blood pH and bicarbonate concentrations, thereby leading to metabolic acidosis.
Interpretation of results: norm, reasons for increased performance
Reference values: 0.5-2.2 mmol/liter. The decrease in indicators is not critical. High levels of this substance can indicate various diseases, including life-threatening conditions. For example, an increase in indicators is observed in acute heart failure, leukemia, circulatory disorders, and diabetes. Only a doctor can make an accurate diagnosis and prescribe treatment. Also, an increase in indicators can be caused by emotional or physical stress, alcohol intoxication. Even in the absence of symptoms, if abnormalities are detected, the patient's condition should be monitored by a doctor.
Indications
- Diagnosis of circulatory pathologies resulting in tissue hypoxia (insufficient oxygen consumption by tissues);
- Assessing the degree of acidosis and prescribing resuscitation measures;
- Detection of diseases of the cardiovascular system;
- Suspicion of non-insulin-dependent diabetes mellitus;
- Determining the cause of lactic acidosis;
- Assessment of the acid-base status of the body and blood pH;
- Diagnosis of asphyxia (severe oxygen deprivation) and enzymopathy (impaired enzyme activity) in newborns;
- Pathological changes in muscles and tissues;
- Differential diagnosis of myopathies (hereditary muscle diseases).
The analysis is deciphered by specialists: endocrinologist, cardiologist, oncologist, traumatologist, surgeon, therapist, pediatrician, etc.
Where does the pain come from?
Then why do muscles hurt the next morning after training? The fact is that under intense loads, the so-called myofibrils are destroyed - thin threads running along the muscle fibers. This is how pieces of dead tissue form in the muscles. The body's immune system finally destroys them and removes them from the body. However, while the destruction process is taking place, free radicals are released in the tissues, and the cells begin to lack water. As a result, pain receptors located on cell membranes are excited, and the person feels pain in the muscles.
BIOLOGICAL CONTROL (MONITORING) IN SPORTS TRAINING[edit | edit code]
Source:
Textbook for Universities “Sports Physiology”. Author
: I.I.
Zemtsova Ed.
: Olympic literature, 2010.
The general goal of biological control in sports is to increase the effectiveness of sports training by optimizing physical activity based on an objective assessment of the athlete’s functional readiness.
At different stages of athletes’ training, there are different tasks, in accordance with which the goal and forms of control are determined. In the theory and practice of sports, there are four main types of control: operational, current, staged and in-depth (Volkov, 1996; Biological control..., 1996; Kurochenko, 2005; Levushkin, 2001; Platonov, 1997; Clausen, 1997).
Operational control (urgent)
involves the assessment of operational states - urgent reactions of the athletes’ body to the load during individual training sessions and competitions.
Current control
is aimed at assessing current conditions resulting from physical activity in a series of classes, training or competitive microcycles.
Stage control
allows you to assess the athlete’s condition, which is a consequence of the long-term training effect at certain stages of preparation.
Advanced Control
carried out once a year for a comprehensive assessment of the athlete’s preparedness and state of health.
Indicators used in accordance with a certain type of control must be informative and reliable and comply with:
- specifics of the sport;
- age and qualifications of the subjects;
- orientation of the training process.
In sports associated with the manifestation of endurance (swimming, rowing, cycling, cross-country skiing, middle and long distance running, etc.), indicators characterizing the state of the cardiovascular and respiratory systems and metabolic processes are mainly studied. Thanks to them, it is possible to most reliably assess the potential capabilities of athletes in achieving high sports results.
In speed-strength sports, where the main task is the ability to demonstrate short-term muscle tension (sprint running, athletics jumping and throwing, weightlifting, certain disciplines of cycling, speed skating, swimming, etc.), indicators characterizing the state of the nervous system are used as means of control. muscular system, central nervous system, speed-strength components of motor function, which are manifested in specific test exercises.
In sports where sporting achievements are predominantly determined by the activity of analyzers, the mobility of nervous processes that ensure accuracy, regularity of movements in time and space (gymnastics, acrobatics, figure skating, diving, all types of sports games, shooting, etc.), in The control process uses a wide range of indicators. They characterize the accuracy of reproducing the temporal, spatial and power parameters of specific movements, the ability to process information and quickly make decisions, the elasticity of skeletal muscles, joint mobility, coordination capabilities, etc. (Belotserkovsky, 2005; Biological control..., 1996; Brgsyun, 2003; Platonov , 1997).
How to avoid pain
Determining the load
City dwellers move much less than they think.
Calculate how active you are and find out how much you need to move. First
, dose the loads, increasing them gradually and systematically.
If the load is selected correctly, there will be less pain or no pain at all. Be sure to familiarize yourself with the basic principles of effective training to avoid not only discomfort the next day after exercise, but also sports injuries. Secondly
, keep your workouts regular - this will help your muscles get used to constant stress.
Thirdly
, if it was not possible to avoid overtraining, take time for full recovery. Healthy sleep, as well as foods containing antioxidants, help speed up the process of breaking down substances that cause muscle pain.
Recounting protons
The first and main source of protons in an actively working muscle cell is considered not to be the synthesis, but the breakdown of ATP, the energy of which is used for contractions and relaxations: ATP + water -> ADP + phosphate + proton. Phosphate itself is capable of serving as a buffer system that softens fluctuations in the acidity of the environment (this is how it works in the body), but it is actively involved in new reactions in the cell, and does not solve this problem very effectively.
Another source of protons is the above-mentioned coenzyme NAD+, which during glycolysis reactions loses a proton, turning into NADH. By the way, a significant part of the protons (as well as phosphate and pyruvate) found in the intracellular environment are transported to mitochondria and used for the processes of oxidative phosphorylation that take place there. Thus, mitochondria can also be called a factor in reducing acidity. But when muscles absorb energy with enormous intensity, converting ATP into ADP, this reaction is stronger than anything acting against it.
Rice. 3.
The balance between the production and use of protons in a working muscle cell. The appearance of protons is associated with ATP hydrolysis and glycolysis reactions. They are consumed in the reactions of creatine phosphate and lactate. In addition, protons bind to inorganic phosphate and buffer compounds in the cytoplasm.
Increased values (lactic acidosis)
- Pathologies of the cardiovascular system: heart failure, cardiogenic shock (acute left ventricular failure), Raynaud's syndrome (severe vascular disease, spasm of small blood vessels);
- Blood flow disorders and diseases of the circulatory system;
- Non-insulin-dependent diabetes mellitus;
- Increased physical activity (usually among professional athletes);
- Tetany (convulsions due to calcium metabolism disorders);
- Tetanus (a bacterial disease affecting the nervous system);
- Epilepsy (pathology of the nervous system, manifested by convulsive seizures with loss of consciousness);
- Hepatitis (viral inflammation of the liver) in acute form;
- Cirrhosis of the liver (an abnormal change in the structure of the organ tissue);
- Oncological processes: lymphoma (cancer of the lymphatic system), leukemia (blood cancer), etc.;
- Poliomyelitis (highly contagious disease of the nervous system, spinal paralysis);
- Tissue hypoxia (oxygen starvation);
- Hypotension (low blood pressure);
- Heavy bleeding;
- Pulmonary insufficiency, hyperventilation (impaired frequency or depth of breathing).
A temporary increase in lactic acid concentration is possible as a result of:
- deficiency of vitamin B1 in the body;
- long-term regular use of alcohol;
- poisoning with chemical elements: ethanol, salicylates, toxins, methanol, etc.;
- dehydration (dehydration of the body);
- pregnancy (in the 3rd trimester the level of lactic acid may increase slightly);
- taking medications: sodium drug, nitroprusside, adrenaline, metformin, fructose or glucose, propylene glycol, methylprednisolone, phenformin, etc.