Without electrolytes, not a single vital process occurs in the human body. They determine the acid-base balance, maintain osmotic pressure in the blood plasma, and perform other important functions. Without electrolytes, cells will stop interacting, muscles will stop contracting, and nerve fibers will stop transmitting signals. Everything will stop. The balance of sodium, potassium and chlorine is a significant indicator of human health, especially the functioning of the heart and kidneys. Program 116 “Electrolyte balance in the body” allows you to determine the general state of health and diagnose electrolyte metabolism disorders that lead to serious consequences - shock, respiratory and heart failure, arrhythmia and cardiac arrest.
The composition of the program is designed in such a way as to determine the main indicators of electrolyte and acid-base balance:
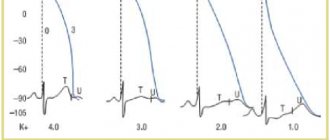
- Potassium (K+) is clinically important for assessing renal function, the cardiovascular system, diagnosing arrhythmias, hypertension, and adrenal insufficiency.
- Sodium (Na+) reflects the health of the kidneys and adrenal glands.
- Chlorine (Cl-). Used for monitoring and dynamic observation of acid-base balance disorders, diagnosis of kidney diseases, diabetes insipidus.
- Calcium (Ca2+) . Determining its concentration is important in diagnosing patients with chronic renal failure, in determining hyperthyroidism caused by phosphate retention in the body, and also for monitoring treatment results.
- Phosphorus . It is used in the diagnosis of various pathological conditions that cause disturbances in phosphorus-calcium metabolism.
- Blood pH – determination of acid-base balance for the diagnosis of acidosis and alkalosis.
Since electrolyte and acid-base imbalances accompany a wide range of acute and chronic diseases, Program 116 “Electrolyte Balance in the Body” can be prescribed to both patients who are already hospitalized and those who have just presented to emergency departments.
Introduction
Asthenic syndrome is the body’s protective reaction to distress of various types, leading to depletion of energy resources, and develops in patients with acute somatic pathology or during an exacerbation of a chronic disease [1].
Asthenia manifests itself as nonspecific complaints of pathological weakness, fatigue (regardless of the type of exercise), insomnia, irritability, inability to relax in combination with muscle pain, tension headaches, dizziness and autonomic dysfunction [2]. The prevalence of asthenic syndrome in general somatic practice varies from 15% to 57%: in patients with chronic somatic diseases it occurs in 45% of cases, in patients who have suffered an acute disease, including an infectious disease, in 55% [3]. Post-infectious asthenic syndrome (PIAS) occurs in 75% of patients after an illness of a bacterial or viral nature.
Clinical manifestations of asthenia appear after 1–2 weeks. after relief of symptoms of an infectious disease and can persist for several months [3, 4]. Their severity depends on the severity of the intoxication syndrome, the presence of complications, age and premorbid background. A mild degree of PIAS is characterized by complaints of weakness, fatigue, weakness, and minor sleep problems. The average severity of PIAS is manifested by pain syndrome (myalgia, arthralgia, cranialgia) and persistent sleep disturbance. With a pronounced degree of PIAS, the patient is unable to perform any physical or mental activity; he experiences tremor, tachycardia, shortness of breath, and, less often, nausea. Difficulties falling asleep and disturbing dreams appear [4]. In the International Classification of Diseases, 10th revision, syndrome G93.3 is separately identified - fatigue syndrome after a viral infection [5].
PIAS is one of the most common and persistent syndromes in patients who have had COVID-19, an infectious disease caused by SARS-CoV-2; its symptoms can persist for 100 days or more after the infection. The “State after COVID-19” (ICD-10 U09.9) [5] is highlighted separately, which is characterized by progressive organ pathology, polyneuropathy, autoimmune disorders and long-term severe asthenic syndrome [6].
Dissociation constant.
According to Arrhenius, the degree of dissociation, i.e. the fraction of molecules that break up into ions increases as the solution is diluted. Assuming that the rates of movement of ions through the electrolyte did not depend on the concentration of the solution, and measuring the electrical conductivity, Arrhenius calculated the degree of dissociation of several electrolytes at different concentrations. W. Ostwald in 1888 used this method to calculate the concentration of free ions and undissociated molecules in solution, and hence the equilibrium constant (dissociation constant) of the dissociation reaction. The reversible dissociation of the electrolyte CA into C+ and A– ions is described by the equation CA C+ + A–, and the dissociation constant is K
= [C+][A–]/[CA] (values in square brackets are concentrations). The last relation satisfactorily describes the behavior of only solutions of weak electrolytes - weak acids and bases. Strong electrolytes, i.e. aqueous solutions of strong acids, bases and most salts behave differently; It turned out that Arrhenius's fundamental postulate about the constancy of ion movement rates and their independence from concentration is not applicable to strong electrolytes.
Arrhenius theory.
Assuming that the electric current in electrolytes was carried by ions, Faraday said nothing about their origin. Some thoughts on this matter were expressed by the German physicist R. Clausius in 1857, and the first most complete description of the process of ion formation belongs to the Swedish physical chemist S. Arrhenius (1883–1897). Arrhenius proposed that salts, acids and bases, when dissolved in a suitable solvent (for example, water), break down (dissociate) into ions. For example, sodium chloride NaCl dissociates into sodium ions Na+ and chlorine Cl–. The electric current does not participate in the dissociation process itself; it only directs the ions to the corresponding electrodes. The theory of electrolytic dissociation not only explains the formation of ions in solution, but also sheds light on many previously unclear phenomena. Thus, in 1887, the Dutch physical chemist J. Van't Hoff discovered that the freezing point of electrolyte solutions is much lower, and the boiling point is much higher than those calculated based on their molecular weights ( see also
SOLUTIONS). The nature of these deviations becomes clear if we consider that the properties of dilute solutions depend not on the nature of the dissolved particles, but on their number. During dissociation, two or more ions are formed from one electrolyte molecule, and the number of particles in the solution becomes much greater than in cases where electrolytic dissociation does not occur for some reason.
Our clinics in St. Petersburg
Structural subdivision of Polikarpov Alley Polikarpov 6k2 Primorsky district
- Pionerskaya
- Specific
- Commandant's
Structural subdivision of Zhukov Marshal Zhukov Ave. 28k2 Kirovsky district
- Avtovo
- Avenue of Veterans
- Leninsky Prospekt
Structural subdivision Devyatkino Okhtinskaya alley 18 Vsevolozhsk district
- Devyatkino
- Civil Prospect
- Academic
For detailed information and to make an appointment, you can call +7 (812) 640-55-25
Make an appointment
Children and newborns are most susceptible to electrolyte imbalance. The concentration of electrolytes in the blood can change significantly when various diseases occur. If the level of electrolytes is elevated, they are removed by dialysis or using special resins; if the level is low, medications are prescribed.
Ways to replenish electrolytes
How to restore electrolytes in the body? The easiest and safest way is to create a balanced diet. It is advisable to select foods that simultaneously contain K, Mg, Ca and Ph in moderate quantities. Don't over-salt your food!
The following foods help maintain an optimal balance of electrolytes in the body:
- apples and pears;
- bananas;
- potato;
- carrot;
- beet;
- broccoli;
- citrus;
- nuts and seeds (especially pumpkin, sesame, flax);
- legumes;
- greens and green leafy vegetables;
- whole wheat bread;
- oat flakes and bran;
- fish, seafood;
- bitter chocolate.
Now about the drinks. Is it worth buying sports? According to manufacturers, isotonic drinks contain 4–6% electrolytes and glucose to replenish energy losses.
As we have already found out, electrolytes are found in many foods. After training, you can drink, for example, 200 ml of orange juice with a pinch of salt, a carrot-apple smoothie or water with dill. There are many recipes for homemade isotonics on the Internet.
Glucose is a beautiful word. But it is a simple carbohydrate made from sugar. You can drink a glass of water with a teaspoon of white powder or eat 3 tangerines. The effect in terms of energy recovery will be exactly the same as from a sports drink.
Pay attention to the composition of industrial isotonics. Some of them contain a lot of sugar, artificial colors, and preservatives. In my opinion, not the best solution for the proper exchange of electrolytes in the body.
Important! Some people drink mineral water to restore electrolytes. However, it is easy to go too far here. Mineral water contains a lot of added salts. You may encounter an oversupply. First, get tested and consult with your doctor. The same goes for dietary supplements with minerals.
Today, in medical centers and laboratories you can take a blood test for electrolytes. The collection comes from a vein. This analysis helps to identify the true cause of poor health. After all, the same symptoms (lethargy, muscle spasms, headaches, dry skin) can occur both due to a lack and due to an excess of various electrolytes.
Electrolytes are charged mineral substances, without which physiological processes in the body do not proceed properly. The best way to maintain optimal fluid and electrolyte balance is to eat healthy foods. Experimenting with mineral water and dietary supplements without consulting a doctor is dangerous. You can drink sports drinks after workouts, but you don't have to. There are healthier analogues from fruits, vegetables and herbs. Watch your diet and your body will work without interruption.
Faraday's laws.
Also on the topic:
ELECTROLYTIC DISSOCIATION. ELECTROLYTES
Electrolysis is the name given to chemical processes occurring under the influence of electric current on electrodes immersed in an electrolyte. The amount of substance formed is related to the amount of electricity passed through the electrolyte (current strength ґ time), Faraday's laws: 1) the amount of substance formed on the electrode when a direct electric current is passed through the electrolyte is directly proportional to the amount of electricity passed, i.e. current strength and electrolysis time; 2) for different electrode processes with the same amount of electricity passed through the electrolyte, the masses of the formed substances are proportional to their chemical equivalents. (The equivalent of an element is the amount of it that combines with 1 mole of hydrogen atoms or replaces the same number of hydrogen atoms in chemical reactions, and the equivalent of a complex substance is the amount that interacts without a residue with 1 equivalent of hydrogen or any other substance. See
. EQUIVALENT MASS.)
Faraday's laws are valid for both solutions and melts and apply to both electrodes. The amount of electricity required to form 1 eq. any substance, the same for all substances; it is equal to 96,485 C and is called the Faraday number or Faraday constant (a fundamental physical constant). This pattern is widely used in practice. Based on the amount of electricity expended, you can calculate the mass or thickness of the metal coating formed during electroplating, and vice versa, by setting the thickness of the coating, you can estimate how much electricity is required for this. Faraday's laws underlie the operation of a voltmeter and instruments designed to measure direct current. see also
ELECTRICAL MEASUREMENTS; ELECTROCHEMISTRY.
Iron
Iron is an electrolyte that ensures the transport and delivery of oxygen to cellular elements and tissues. As a result, the blood is saturated with oxygen, the process of cellular respiration and the formation of red blood cells in the bone marrow are normalized.
Iron enters the body from the outside, is absorbed in the intestines and spreads through the bloodstream throughout the body. Sources of iron are: bran bread, shrimp, crab meat, beef liver, cocoa, egg yolk, sesame seed.
Iron in the body of newborns and children up to one year old varies between 7.16 - 17.90 µmol/l, in children from one to 14 years of age - 8.95 - 21.48 µmol/l, in adults - 8.95 - 30, 43 µmol/l.
People with iron deficiency develop iron deficiency anemia, the immune defense and general resistance of the body decrease, fatigue increases, and fatigue quickly occurs. The skin becomes pale and dry, muscle tone decreases, the digestion process is disrupted, and appetite disappears. Characteristic changes are also noted in the cardiovascular and bronchopulmonary systems: increased heart rate, difficulty breathing, shortness of breath. In children, the processes of growth and development are disrupted.
Women need iron more than men. This is due to the loss of a certain part of the element during monthly bleeding. During pregnancy, this is especially important, since two organisms need iron at once - the mother and the fetus. Special medications will help future mothers and nursing women prevent iron deficiency in the body - “Hemofer”, “Sorbifer”, “Maltofer Fol”, “Heferol” (all drugs are prescribed by a doctor!)
Iron electrolytes in the blood are increased with:
- Hemochromatosis,
- Hypo- and aplastic anemia,
- B12-, B6- and folate deficiency anemia,
- Violation of hemoglobin synthesis,
- Inflammation of the glomeruli of the kidneys,
- Hematological pathologies,
- Lead intoxication.
The causes of blood iron deficiency are:
- Iron-deficiency anemia,
- Lack of vitamins
- Infections,
- Oncopathology,
- Massive blood loss
- Gastrointestinal dysfunctions,
- Taking NSAIDs and glucocorticosteroids,
- Psycho-emotional stress.
Chlorine
Chlorine is a blood electrolyte, the main anion that normalizes water-salt metabolism “paired” with positively charged cations of sodium and other elements (including potassium). It helps to equalize blood pressure, reduce tissue swelling, activate the digestion process, and improve the functioning of hepatocytes.
The level of chlorine in the blood for adults ranges from 97 to 108 mmol/l. For children of different ages, the range of normal values is slightly wider (From 95 mmol/l for most age groups and up to 110-116 mmol/l. The most chlorine can be contained in the blood of newborns).
An increase in chlorine levels (hyperchloremia) develops when:
- Dehydration,
- Alkalose,
- Kidney pathologies,
- Excessive functioning of the glandular cells of the adrenal glands,
- Vasopressin deficiency in the body.
The causes of hypochloremia are:
- Vomit,
- Hyperhidrosis,
- Treatment with large doses of diuretics,
- TBI,
- Acidotic coma,
- Regular intake of laxatives.
Patients with hypochloremia experience hair and teeth loss.
Table salt, olives, meat, dairy and bakery products are rich in chlorine.
Stock
Antibody test for coronavirus (COVID-19)
from 2000 a
Rapid test for coronavirus (COVID-19)
Results within 25 minutes from the moment the biomaterial is submitted
1600a
Coronavirus (COVID-19) antibody test at home
from 4200 a
Coronavirus (COVID-19) test at home
from 4950 a
Coronavirus (COVID-19) test
2750a