When hemoglobin breaks down in sideroblast cells, hemosiderin is formed - a pigment containing iron molecules. It is deposited in the structures of the bone marrow, in the liver, in the spleen, in the sweat and salivary glands and in other organs. The main function of the pigment is the deposition and transfer of oxygen, as well as iron molecules, and participation in the transformation of biochemical complexes. With certain changes in the body, the synthesis of iron-containing compounds is activated.
Hemosiderosis (capillaritis, hemorrhagic pigmentary dermatosis) is a disease from the category of pigmentary dystrophies associated with metabolic disorders and excessive accumulation of hemosiderin in tissues and blood vessels. The skin and internal organs are affected. Deposits of hemoglobinogenic pigment can be widespread or local. Establishing the nature of the disease is difficult. Treatment is carried out by dermatologists, pulmonologists, hematologists, immunologists and other specialists.
About the reasons for the development of hemosiderosis
Skin hemosiderosis can be primary or secondary, developing due to injuries and skin infections.
The causes of primary skin capillaritis may be the following factors:
- vascular changes due to the presence of hypertension, varicose veins, etc.
- various endocrine disorders - the patient has diabetes mellitus and other pathologies.
Secondary hemosiderosis of the dermis can be caused by:
- scratching the skin, damage, injuries;
- skin diseases – dermatitis, neurodermatitis, etc.;
- pustular skin diseases.
Risk factors that can provoke the development of the disease include:
- frequent hypothermia;
- stress;
- excessive physical activity;
- taking diuretic, anti-inflammatory and antibacterial drugs.
Generalized hemosiderosis develops against the background of previous diseases. Most often, the main causes are systemic blood diseases, autoimmune diseases, severe intoxications and exposure to infectious agents. Increased deposition of hemosiderin may be due to:
- leukemia;
- sepsis, malaria and other infections;
- the presence of Rh conflict;
- liver damage;
- constant blood transfusions;
- taking large doses of sulfonamides, drugs containing quinine or lead.
Why iron-containing pigment accumulates in the lungs is not entirely clear. Often the reasons lie in genetic predisposition, in congenital anomalies of the pulmonary capillaries, in existing heart diseases.
Principles of treatment
To date, it is believed that not a single drug has a 100% evidence base for its effectiveness in treating hemosiderosis. Thus, the treatment of these diseases is a problem and a challenge for doctors. However, some hemosiderosis can resolve on its own and then unexpectedly recur.
Corticosteroids are effective in reducing inflammation and suppressing itching in some hemosideroses. Antihistamines inhibit endogenous histamine production and also reduce the feeling of itching in patients.
The administration of griseofulvin, cyclosporine A, vitamin C and bioflavonoids has certain benefits. There is evidence of the positive effect of PUVA therapy (psoralen (P) + ultraviolet A (UVA) = PUVA or PUVA) - irradiation of the skin with ultraviolet light A with preliminary intake of a photosensitizer (psoralen) for greater effectiveness of light exposure.
hardware techniques can be used aimed at selective destruction of the pigment. For local destruction of hemosiderin for aesthetic purposes, IPL therapy can be used: a method in which high-intensity pulsed light destroys the pigment, making it more accessible for evacuation during phagocytosis.
Questions from our users:
- skin hemosiderosis laser treatment
- hemosiderosis of the skin after injections
- hemosiderosis of the skin in adults
- local and general hemosiderosis causes
Characteristic symptoms
Clinical manifestations depend on where the hemoglobinogenic pigment accumulates.
Pulmonary hemosiderosis
Idiopathic (primary) pulmonary hemosiderosis is manifested by the following symptoms:
- moist cough;
- elevated temperature, fever;
- presence of shortness of breath and respiratory failure;
- cough with blood;
- chest pain, severe dizziness;
- pale skin;
- hypochromic anemia;
- skin cyanosis;
- enlarged spleen and liver;
- drop in blood pressure, heart rhythm disturbance.
Brown purpura of the lungs, usually found in children and young adults, is a serious disease. With a prolonged course of the disease, recurrent infarction-pneumonia and other pathologies very often develop.
At CELT you can consult a pulmonologist.
- Initial consultation – 3,500
- Repeated consultation – 2,300
Make an appointment
Skin hemosiderosis
The following symptoms are characteristic:
- the appearance of brown pigment spots in the lower extremities and other parts of the body;
- hemorrhagic rash on the skin of the hands, feet, forearms, etc.;
- slight itching in problem areas;
- formation of plaques, nodules and papules.
Chronic hemosiderosis of the dermis is usually diagnosed in men over 30 years of age. The disease occurs without damage to internal organs.
At CELT you can consult a dermatologist.
- Initial consultation – 3,500
- Repeated consultation – 2,300
Make an appointment
Purpurative pigmentary dermatosis of the liver is manifested by enlargement of the organ, its soreness, yellowness of the integument, and the presence of a pigmented rash on the hands, in the armpits, and on the face. If the kidneys are damaged, swelling in the legs, dyspepsia, changes in taste, lower back pain, etc. appear.
Idiopathic pulmonary hemosiderosis is a rare disease that occurs mainly in childhood. The article presents literature data and describes a clinical case of idiopathic pulmonary hemosiderosis in a 14-year-old child.
Idiopathic pulmonary hemosiderosis
Idiopathic pulmonary hemosiderosis is a rare disease that occurs primarily in children. The article presents the published data and described a clinical case of idiopathic pulmonary hemosiderosis in a child of 14 years.
Idiopathic pulmonary hemosiderosis (brown induration of the lungs, Delen-Gellerstedt syndrome, “iron lung”) is a rare disease that is characterized by hemorrhage in the lungs and a wavy, relapsing course.
Studying the epidemiology of idiopathic pulmonary hemosiderosis is complicated by the extreme rarity of this disease. Its frequency ranges from 0.24 to 1.23 per 1,000,000 population [2, 9, 10].
There are several assumptions regarding the etiology and pathogenesis of this disease. Opinions have been expressed regarding congenital structural disorders, increased permeability of the vascular wall of the pulmonary capillaries, the presence of abnormal anastomoses between the pulmonary arteries and veins [2], and a hereditary predisposition cannot be excluded [2, 4]. The immunopathological nature of the disease is supported by the presence of such a form as Heiner's syndrome - idiopathic pulmonary hemosiderosis with increased sensitivity to cow's milk [1, 7, 8], as well as the response to therapy with glucocorticosteroids and immunosuppressants [3]. The clinical picture of idiopathic pulmonary hemosiderosis during exacerbation is characterized by cough, hemoptysis, body temperature rises, shortness of breath, cyanosis, and moist rales are heard in the lungs [1-3]. Hematological disorders are manifested by iron deficiency anemia with a hemolytic component, which is caused by hemolysis of red blood cells released from damaged capillaries [5]. Diagnostically significant is the detection of siderophages in sputum or tracheal aspirate, as well as in some cases in gastric lavage waters [1, 6]. A study of external respiration function reveals predominantly restrictive ventilation disorders. The X-ray picture varies from a decrease in the transparency of the pulmonary fields, the appearance of multiple small focal ones to the appearance of large infiltrate-like shadows. Characteristic features of the X-ray picture are sudden onset and relatively rapid reverse dynamics. Frequent exacerbations of the disease lead to the development of interstitial pneumosclerosis [2]. Treatment of patients with hemosiderosis involves the prescription of corticosteroid drugs, and if ineffective, cytostatic drugs (azathioprine, cyclosporine A) [11]. According to Saeed MM et al., who summarized the experience of observing 17 children suffering from idiopathic pulmonary hemosiderosis, the five-year survival rate exceeds 80% [11].
Considering that pulmonary hemosiderosis is a fairly rare disease, below is a case of clinical observation from our own practice.
Patient R., 14 years old, was admitted to the pulmonology department of the Republican Children's Clinical Hospital (RCCH) with complaints of weakness, dizziness, wet cough with the discharge of sputum streaked with blood. I felt a deterioration in my condition about two weeks before hospitalization.
At the time of admission, the child had been ill for two years, when he was first admitted to the Russian Children's Clinical Hospital for examination for moderate iron deficiency anemia. About 4 months later, he consulted a pediatrician with complaints of cough and weakness. With a diagnosis of pneumonia, he was hospitalized in the pulmonology department of the Russian Children's Clinical Hospital, where, as a result of an examination, a general blood test revealed hypochromic anemia of moderate severity (hemoglobin 86 g/l), in the sputum - siderophages characteristic of damage to the interstitial tissue of the lungs, changes on a computed tomogram of the chest organs cells and a diagnosis was made: Idiopathic pulmonary hemosiderosis. Iron deficiency anemia of moderate severity. He was regularly observed by a pulmonologist and was treated with glucocorticosteroids (oral prednisolone). At the time of this hospitalization, he had not taken prednisolone for a year.
A child from 2 pregnancies, 2 term births (the first birth ended in stillbirth) by cesarean section with a weight of 2450, a height of 49 cm, an Apgar score of 7-8 points. He grew and developed according to his age. Vaccinations were done according to the calendar. History of rare acute respiratory diseases.
Upon admission, the condition was serious due to the disease, the state of health was moderate. The child is lethargic. Reduced nutrition, proper physique. Weight 37 kg. The skin is pale, dry. The sclera is subicteric. The pharynx is not hyperemic. The cervical and submandibular lymph nodes were palpated, measuring 0.5 cm, soft, elastic, painless. The chest is barrel-shaped. Percussion - dullness in the lower fields of the lungs. Auscultation of the lungs reveals weakened breathing, intermittent moist fine-bubble rales. Respiration rate is 22-24 per minute. The limits of relative cardiac dullness corresponded to the age norm. Heart sounds are clear, the rhythm is correct. Heart rate 90 beats per minute. The abdomen is soft and painless. The liver is at the edge of the costal arch. The spleen was not palpable. Stool and urination are not impaired.
On the 9th day of hospitalization, despite the therapy (systemic glucocorticosteroids, antibacterial therapy, symptomatic therapy), the child experienced a deterioration in his condition, an increase in body temperature to 39-400C, severe weakness, and shortness of breath. The child was transferred to the intensive care unit, where he was on artificial ventilation for 4 days.
Upon admission, a general blood test revealed hypochromic anemia of severe mixed etiology. The number of erythrocytes in the general blood test was 1.99x1012/l, hemoglobin - 45 g/l, hemoglobin content in an erythrocyte - 22.6 pg, hemoglobin concentration in an erythrocyte - 28.1%, erythrocyte volume - 80.4 fl, reticulocyte count – 7.7 0/00. A blood test was performed for iron content, which was 1.85 µmol/l (normal 12.5-32 µmol/l), latent iron-binding capacity - 67 µmol/l (normal 20-62 µmol/l), total iron-binding capacity - 68.9 µmol/l (normal 44.8-76.1 µmol/l), transferrin - 3.04 g/l (normal 1.83-3.63 g/l), transferrin saturation percentage - 2.4% at normal 15-50%, ferritin 66 µg/l (normal 7-140 µg/l).
In addition, on the fourth day of hospitalization, there was an increase in leukocytes to 17.7x109/l, neutrophilia - band 5%, segmented 74%, with a subsequent increase in neutrophilia to 94%. In a biochemical blood test, an increase in hypoproteinemia was noted to 42 g/l (normal 66-87 g/l), hyperbilirubinemia to 67.8 µmol/l (normal 1.7-20.5 µmol/l) due to the indirect fraction. On the 12th day from the moment of hospitalization - acceleration of ESR to 30 mm/h, thrombocytopenia to 100x109/l.
Immunological examination upon admission revealed a decrease in the level of IgG to 4.8 g/l (normal 8-16.4 g/l), a reduced level of CD3+ cells to 28% (normal 52-76%), CD4+ - to 11% (normal 31-46%), CD8+ - up to 21% (normal 23-40%), CD16+ - up to 3% (normal 9-19%), CD25+ - up to 7% (normal 10%). Hyperergic type of chemiluminescent curve.
The blood is negative for HIV infection. Throat smear for flora – single S. aureus, C. albicans. The electrocardiogram shows sinus rhythm with a heart rate of 81-83 per minute. The electrical axis of the heart is not deviated. Incomplete blockade of the right bundle branch. Slows down the electrical systole by 0.06 sec. Echocardiography revealed a congenital heart defect - atrial septal defect, secondary, mitral regurgitation 1+. Slit-like defect of the interventricular septum in the upper third. The cavity of the left ventricle is at the maximum limit of normal. RPH 25 mm Hg. Art.
Study of the gas composition of arterial blood: pH 7.33, pCO2 34 mm Hg. st, pO2 33.3 mm Hg. Art., SaO2 55.2%.
Other indicators of a biochemical blood test (urea, creatinine), urine and feces tests are within the age norm, ultrasound examination of the abdominal organs and kidneys is without any features.
A chest x-ray (CH) at the time of admission revealed focal-like shadows of a confluent nature throughout all pulmonary fields. The pulmonary pattern is enriched, mainly due to the interstitial component. The contours of the mediastinum and the contours of the domes of the diaphragm are not clearly visible.
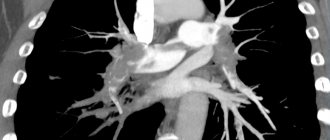
Computed tomography of the OGK revealed a significant decrease in pneumatization of the pulmonary fields. At all levels there are multiple, small-focal, merging with each other into extensive conglomerates, infiltrate-like compactions, with a density reaching up to -370 units N. The pulmonary pattern is enriched, in places deformed, with symptoms of local pneumofibrosis. Strengthening, thickening of the interlobular, peribronchial, perivascular interstitium. Reaction of costal and interlobar pleura. Pleurocostal, pleuromediastinal, supraphrenic adhesions. Enlargement of intrathoracic lymph nodes up to 7-12 mm (Fig. 1, 2, 3).
Figure 1. Overview topogram of the chest organs in a direct projection. Strengthening, deformation of the pulmonary pattern. Periprocess along the bronchovascular structures
Figure 2. Axial CT image of the chest at the level of the tracheal bifurcation. Enlargement of the lymph nodes of the central mediastinum. The retrosternal space of the anterior mediastinum is filled with tissue of the thymus gland
Figure 3. Axial CT image of the chest in the basal-phrenic regions. Multiple small focal compactions merging with each other and forming extensive infiltrate-like overlays
Against the background of the treatment: systemic glucocorticosteroids (pulse therapy, then oral prednisolone), massive antibacterial therapy, infusion therapy, symptomatic therapy, on the 30th day from the moment of hospitalization, clinical stabilization was achieved, the child’s well-being significantly improved. The hemogram indicators (leukocytes 14.6x109/l, erythrocytes 3.54x1012/l, hemoglobin 82 g/l, platelets 370x109/l, ESR - 5mm/h), biochemical blood parameters (total protein 60.7 g/l, total bilirubin 4 µcol/l), positive X-ray dynamics were noted (foci-like shadows disappeared, the pulmonary pattern began to be differentiated more clearly).
On the 42nd day from hospitalization, the child was discharged home with improvement. It is recommended to take systemic glucocorticosteroids on a tapering schedule. Follow-up observation for more than six months demonstrated the absence of exacerbation of the disease in the child.
In conclusion, it must be added that idiopathic pulmonary hemosiderosis is a disease that is difficult to diagnose and has a serious prognosis. It is often diagnosed only after unsuccessful attempts at antibacterial therapy for “pneumonia.” Timely diagnosis of the disease and the prescription of adequate immunosuppressive therapy can prevent the rapid progression of the process and the development of pneumosclerosis.
D.V. Kazymova, E.N. Akhmadeeva, D.E. Baykov, G.V. Baykova
Bashkir State Medical University, Ufa
Republican Children's Clinical Hospital, Ufa
Akhmadeeva Elza Nabiakhmetovna - Doctor of Medical Sciences, Professor, Head of the Department of Hospital Pediatrics with a course of outpatient pediatrics
Literature:
1. Artamonov R.G. Idiopathic pulmonary hemosiderosis // Medical scientific and educational journal. - 2002. - No. 6. - P. 189-193.
2. Bogorad A.E., Rozinova N.N., Sukhorukov V.S. and others. Idiopathic hemosiderosis of the lungs in children // Russian Bulletin of Perinatology and Pediatrics. - 2003. - No. 4. - P. 29-35.
3. Davydova V.M. Interstitial lung diseases // Practical medicine. Pediatrics. - 2010. - No. 45. - P. 22-28.
4. Fadeeva M.L., Gingold A.M., Shchegoleva T.P. and others. Idiopathic hemosiderosis of the lungs in children // Issues. ocher mat. and children - 1976. - No. 4. - P. 37-42.
5. Yatsenko E.A., Plakhuta T.G., Guzheedova E.A. Iron deficiency anemia as a manifestation of isolated pulmonary hemosiderosis // Pediatrics. - 1991. - No. 6. - 87 p.
6. Cohen S. Idiopathic pulmonary hemosiderosis // Am. J. Med. Sci. - 1999. - Vol. 317. - P. 67-74.
7. Heiner DC, Sears JW, Kniker WT Chronic respiratory disease associated with multiple circulating precipitins to cow's milk // Am. J. Dis. Child. - 1960. - Vol. 100. - P. 500-502.
8. Heiner DC, Rose B. Elevated levels of YE (IgE) in conditions other than classical allergy // J. Allergy. - 1970. - Vol. 45. - P. 30-42.
9. Kjellman V., Elinder G., Garwicz S. et. al. Idiopathic pulmonary hemo¬siderosis in Swedish children // Acta Pediat. Scand. - 1984. - Vol. 73. - P. 584-588.
10. Ogha S., Takahashi K., Miyazaki S. et. al. Idiopathic pulmonary he¬mosiderosis in Japan: 39 possible cases from a survey questionnaire // Eur. J. Pediat. - 1995. - Vol. 154. - P. 994-995.
11. Saeed MM, Woo MS, MagLaughlin EF et. al. Prognosis in pediatric idiopathic pulmonary hemo¬siderosis // Chest. - 1999. - Vol. 116. - P. 712-725.
Diagnostics
A dermatologist examines the patient, examines the nature of the rash and the presence of characteristic signs. Laboratory tests are required - blood biochemistry with determination of iron levels, general urine tests, PCR tests. The content of sputum and urine is analyzed using a desferal test. To clarify the diagnosis, the affected segments are sent for a biopsy. Histological studies of the skin, bone marrow, lungs, lymphatic tissues, liver, and kidneys help detect pigment deposits. For diagnostic purposes, bronchoscopy with examination of lavage water can be performed, a chest x-ray, MRI, and computed tomography may be performed.
Therapeutic tactics of hemosiderosis
Treatment is prescribed by a dermatologist and depends on the clinical manifestations and severity of the disease.
Conservative therapy:
- Prescription of glucocorticosteroids. These are first-line drugs that suppress inflammation, restore physiological processes, stop autoimmune processes and stabilize cell walls. In 50% of cases, drugs cure hemosiderosis.
- Immunosuppressants in combination with plasmapheresis. Cytostatics and other immunosuppressants suppress the immune system, including at the cellular level, and prevent the formation of new antibodies. The blood is filtered using a special apparatus. Toxins, accumulated antibodies and immune complexes that increase vascular permeability are removed from tissues, vessels and cells.
- Vitamin complexes containing vitamin C, rutin, calcium, etc.
- Iron supplements in the presence of anemia and other complications.
- Hemostatic drugs.
- Medicines to eliminate symptoms of the disease. If necessary, bronchodilators, angioprotectors, anticoagulants, antiplatelet agents, etc. are prescribed.
- Corticosteroid ointments are prescribed locally.
- Inhalations with oxygen.
- Cryotherapy.
In severe cases, doctors perform a splenectomy, and patients have their spleen removed to achieve stable remission. They resort to blood transfusion, PUVA therapy, and prescribe drugs that help remove iron from the body.
Complications
If a patient with capillaritis does not receive proper treatment, dangerous complications develop that can lead to disability and death. Advanced forms of pulmonary hemosiderosis, when the pathological process affects the alveoli, are especially difficult to treat.
Possible complications:
- extensive pulmonary infarction;
- acute respiratory failure;
- multiple internal hemorrhages;
- constant overload of the heart and the formation of cor pulmonale;
- persistent increase in blood pressure;
- spontaneous pneumothorax.
Hemosiderosis of the skin has a favorable course. In advanced forms of the disease, cosmetic defects appear that require proper correction.
Pathogenesis
Macrophages, as well as histiocytes , epithelial cells , and endotheliocytes with the help of their sideroblasts are capable of synthesizing hemosiderin molecules. They are found in the spleen, liver, lymph nodes, bone marrow and other organs. The simplest example of local accumulation of pigment is a common hematoma .
Usually, the process of local hemosiderolysis is triggered by a massive hemorrhage or many diapedetic hemorrhages, where extravascular (extravascular) hemolysis occurs in the presence of oxygen molecules.
hemotoidin is formed , and hemosiderin is formed only in the periphery for about 2-3 days. Its presence in the tissue means that the hemorrhage is old.
Normally, the pigment accumulated in cells does not have a damaging effect on tissues or the structure and functioning of organs, but if hemosiderin accumulates against the background of sclerotic processes, this can lead to functional disorders and consequences. For example, the formation of a cyst in the brain: since hemosiderin is deposited between healthy tissues and blood clots at the site of hemorrhage, repeated hemorrhage leads to the formation of a cyst with brown edges.
The general excessive accumulation of hemosiderin in the body causes excess iron during intravascular (intravascular) hemolysis or it is associated with increased absorption of iron from consumed foods. In this case, siderophages do not have time to absorb hemosedirene and, as a result, fibers, intercellular substance and tissues leak iron and give the spleen, liver, bone marrow, and lymph nodes a brown color. Deposits of hemosiderin can be found in the epithelial cells of the sweat glands, salivary glands and kidneys. bilirubin accumulate . Due to increased bilirubin, patients may experience hemolytic jaundice .
Bone marrow hemosiderosis
A fairly common phenomenon is when pigment molecules do not damage parenchymal cells and there are no organ dysfunctions, but if an increased amount of iron has a damaging effect and causes atrophy of the parenchyma, sclerosis , which reduces and disrupts its functioning, then we are talking about a hereditary disease - hemochromatosis .