Pharmacological properties of the drug Fozinopril
ACE inhibitor. Reduces the formation of angiotensin II from angiotensin I, which leads to a decrease in peripheral vascular resistance and systemic blood pressure. Suppresses the synthesis of aldosterone in the adrenal glands. The onset of therapeutic action is observed 1 hour after oral administration, the maximum reduction in blood pressure is achieved within 3–6 hours. The hypotensive effect persists for 24 hours, however, it may take 2–3 weeks to achieve a stable hypotensive effect in some patients. During long-term treatment, the effect persists. After oral administration, about 36% of fosinopril is absorbed in the digestive tract; the degree of absorption does not depend on food intake. Hydrolysis of fosinopril with the formation of pharmacologically active fosinoprilat occurs in the mucous membrane of the digestive tract and partially in the liver. The maximum concentration of fosinoprilat in blood plasma is achieved after 3 hours. Plasma protein binding is 95%. Does not penetrate the BBB. Fosinoprilat is excreted unchanged in urine and bile. The half-life is 11.5 hours. In patients with renal failure, there is no noticeable change in the pharmacokinetics of fosinopril due to a compensatory increase in its excretion by the liver. In patients with impaired liver function, a slight decrease in the hydrolysis of fosinopril is possible. There is evidence of a compensatory increase in the excretion of fosinopril by the kidneys with a simultaneous decrease in the hepatic clearance of fosinopril in this category of patients.
Introduction
In the new millennium, the problems of arterial hypertension (AH) and chronic heart failure (CHF) have become unexpectedly acute. These terrible diseases are connected by a common cause-and-effect relationship. It has been proven that hypertension is one of the most important causes of the development of CHF. According to the Framingham study, among the risk factors for CHF, such as left ventricular (LV) hypertrophy, previous myocardial infarction (MI), angina pectoris, diabetes mellitus (DM), valvular heart disease, high blood pressure (BP) is the most accurate predictor of the development of this pathological condition [1]. In Western countries, the role of hypertension as a cause of the development of CHF becomes leading in older age groups, leading to disruption of diastolic and subsequently systolic myocardial function. In our country, hypertension occupies 80% of the causes of CHF and contributes to its decompensation mainly due to diastolic dysfunction [2]. Thus, according to the Russian epidemiological study EPOCHA-O-CHF, the proportion of outpatients with CHF and preserved LV ejection fraction (EF) (> 40%) exceeds 80% [3]. This is not associated with an increase in the number of elderly people, as in Europe, but with the widespread and ineffective treatment of hypertension. In the European EuroHeart Survey HF, the same figure is 53% [4].
At present, there is no doubt about the direct relationship between the pathogenetic mechanisms of development and progression of hypertension and CHF. Cardiac decompensation is one of the most dangerous complications of hypertension. In hypertension, hemodynamic overload leads to remodeling of both the heart and peripheral vessels. As a result, a hypertensive heart is formed, which is manifested by the development of diastolic and then systolic dysfunction of the LV with a decrease in cardiac output. Remodeling of peripheral vessels is accompanied by an increase in pre- and afterload, and the activation of renal mechanisms for the development of CHF. From the modern perspective of the cardiovascular continuum, remodeling of the cardiovascular system is considered, firstly, as a complication of hypertension and a harbinger of clinical manifestations of CHF, and secondly, as a factor in the progression of hypertension and a predictor of cardiac decompensation [5]. Taking into account that hypertension is one of the main risk factors of the cardiovascular continuum, and CHF is one of its final stages, successful treatment affecting their common pathogenetic basis, i.e., remodeling, can be considered as prevention of the development of cardiac decompensation . In addition to hemodynamic mechanisms, an important role in the progression of both hypertension and CHF is played by chronic hyperactivation of neurohormonal systems, which is not only a connecting link in the pathogenesis of both diseases, but also the main component of remodeling processes [6]. Therefore, in the treatment of both pathologies, the use of neurohormonal modulators, primarily angiotensin-converting enzyme inhibitors (ACEIs), is most justified.
A real boom in the study of various aspects of hypertension and heart failure, as well as multicenter placebo-controlled clinical trials of drugs, have led to a radical revision of national and international recommendations for the diagnosis and treatment of hypertension and heart failure. But, despite numerous publications and monographs published in recent years and devoted to these problems, practicing physicians still experience certain difficulties when choosing medications, especially when several cardiovascular diseases are combined, such as hypertension and CHF.
The role of ACE inhibitors in the treatment of hypertension and heart failure
ACEI in the 21st century remain one of the most effective means of treating cardiac patients. The benefits of their use in the prevention of cardiovascular diseases, including CHF, in patients both with and without hypertension have been convincingly proven. Treatment with ACE inhibitors in patients with hypertension is associated with a reduction in the risk of developing CHF by an average of 16% [7]. This class of drugs occupies a leading position among drugs for the treatment of CHF, exerting a modulating effect on the general pathophysiological mechanisms of hypertension and CHF by reducing the activity of the renin-angiotensin-aldosterone system (RAAS) by blocking the formation of active angiotensin II (A-II), as well as activation bradykinin system. Assessing the role of ACE inhibitors in the treatment of CHF, Braunwald E. (1991) called them “the cornerstone in the treatment of CHF,” alpha Cohn J. (1998) called them “the gold standard of therapy.” The quarter of a century that has passed since the creation of the first ACE inhibitor, captopril, is now called the “era of ACE inhibitors.” The pharmacological effects of ACEIs in hypertension and CHF are qualitatively the same. At the same time, arterial vasodilation with a decrease in afterload and blood pressure plays an important role in hypertension, and venous vasodilation with a decrease in preload in CHF. Slowing down cardiac remodeling processes is of particular importance in both diseases. For hypertension, first of all, it is necessary to improve diastolic function, for CHF - both diastolic and systolic function of the LV. The nephroprotective properties of ACE inhibitors are important in both pathological conditions, and the antiarrhythmic effect of this class of drugs is important in CHF.
The effectiveness of ACE inhibitors has been proven for any degree of severity (functional class - FC) of CHF: studies SOLVD-prevention (I FC), SOLVD-treatment (II FC), V-HeFT II (III FC), CONSENSUS (IV FC). ACEIs have been shown to reduce the risk of death in patients with CHF by 23%. They are first-line drugs for the treatment of CHF with LV systolic dysfunction, since this is the only group of drugs that can not only reduce the manifestations of CHF, but also significantly slow down the progression of remodeling and improve long-term prognosis [6]. The dominant position of ACE inhibitors in preventing and slowing down LV remodeling in patients with diastolic dysfunction has been confirmed (PREAMI study) [8]. They have a positive effect on such remodeling parameters as indices of sphericity and relative wall thickness, myocardial stress [9], and contribute to the maintenance of normal EF and LV myocardial mass [10] by inhibiting the activation of the RAAS, stabilizing the level of bradykinin and the release of nitric oxide [11]. A more significant regression of LV hypertrophy during treatment with ACE inhibitors compared to other drugs has also been shown [12], due not only to a decrease in systemic blood pressure, but also to their antiproliferative effect on cardiomyocytes and myocardial fibroblasts. Since the rhythm rate, cardiac output and pulmonary capillary wedge pressure do not change significantly during the use of ACEIs, they suggest a likely effect directly on myocardial relaxation. The effect of improving diastolic function under the influence of ACE inhibitors is associated with a decrease in hypertrophy and an increase in “compliance” of the LV walls, a decrease in afterload and suppression of abnormal collagen formation in the interstitium through neurohumoral effects (decreased activity
A-II, aldosterone, blocking bradykinin degradation, improving endothelial function). However, a number of questions regarding the mechanisms of their positive effect in hypertension and CHF remain open.
A series of studies (AIRE, SAVE, SOLVD, TRACE, V-HeFT, etc.) have shown the anti-ischemic effect of ACE inhibitors. Its mechanisms are still being clarified, but a certain role is assigned to slowing down the remodeling of the coronary arteries, improving subendocardial perfusion with a decrease in LV end-diastolic pressure or changing neurohormonal influences, stabilizing atherosclerotic plaques and improving endothelial function due to the direct tissue effect of ACE inhibitors [13, 14]. The experiment demonstrated the possibility of new formation of the capillary network in the myocardium due to the influence of ACE inhibitors on the metabolism of bradykinin.
It is believed that in patients with CHF with preserved systolic function, as a rule, there is no noticeable increase in the systemic level of renin and A-II, but there is activation of the local RAAS, therefore ACE inhibitors with high affinity for tissue ACE are promising [13]. However, there is currently little evidence of the effectiveness of ACEIs in diastolic CHF. Only a few studies (HOPE, EUROPA) confirmed their effect in patients with coronary artery disease without LV dysfunction [7, 14], and the effect on LV diastolic filling in patients with CHF was studied only in small clinical studies. At the same time, the authors more often noted the beneficial effect of ACE inhibitors in the form of redistribution of blood flow with an increase in the early phase and a decrease in the late phase of diastole, which indicates an improvement in the active relaxation of the LV myocardium [15, 16].
At the same time, there are indications of opposite changes in transmitral diastolic flow [10] and even the absence of positive dynamics of diastolic indices during long-term therapy with enalapril, captopril, lisinopril, perindopril and other ACE inhibitors [17, 18]. A likely explanation for such discrepancies may be the multidirectional nature of changes in transmitral diastolic flow due to the presence initially of not only “hypertrophic”, but also pseudonormal or restrictive types of diastolic dysfunction. It is also believed that the effectiveness of ACE inhibitors on myocardial hypertrophy and diastolic relaxation depends on the presence of the ACE polymorphism gene in patients [19].
The problem of optimal choice of ACEI
Due to the presence of various ACE inhibitors on the market (12 original molecules of different drugs of this class are registered in Russia), the question arises about their optimal choice. At the same time, to justify the benefits of a particular ACE inhibitor, a thoughtful and comprehensive assessment by the physician of the range of not only pharmacological properties, but also the available evidence of its clinical effectiveness and safety is necessary. An “ideal” ACEI should combine high efficacy, proven in clinical studies, maximum safety, and have a minimum of side effects. It is known that the choice of ACE inhibitors is greatly influenced by the doctor’s habit and faith, socio-economic factors (including the cost of treatment), the severity of the patient’s condition, individual tolerance, etc.
Although no fundamental differences were found between individual ACE inhibitors, a significant effect on the symptoms of the disease, quality of life, prognosis and safety in CHF was proven in international studies only for six drugs (captopril, enalapril, fosinopril, lisinopril, ramipril, perindopril) and for two more (spirapril , quinapril) – in the multicenter Russian programs VNOK and OSSN. This review analyzes the available data assessing the pharmacological and clinical features of fosinopril, due to which it has a number of advantages over other ACE inhibitors in the treatment and prevention of CHF in patients with hypertension. Not long ago, the drug Fosicard® (Actavis company) appeared on the domestic pharmaceutical market, which became the first high-quality generic analogue of fosinopril. The high quality, effectiveness and safety of Fosicard® are confirmed by the results of a study assessing its bioequivalence to the original fosinopril. Despite the fact that this drug is a typical representative of the ACEI class, it has a certain structural feature - it contains a phosphonyl group in its chemical formula. This feature gives it a number of unique properties that distinguish it favorably from other drugs of this class and allow it to be classified as the third (most modern) generation of ACE inhibitors [20].
Clinical and pharmacological properties of fosinopril
Fosinopril is a prodrug, that is, it acts after absorption and transformation into an active metabolite - fosinoprilat, which circulates in a state bound to blood plasma proteins (95-98%) with a half-life (in healthy individuals) of about 12 hours. The advantages of fosinopril include high affinity for lipids - the lipophilicity index of fosinoprilat is more than 2.0 units, while for perindoprilate it is 0.872; and for enalaprilat – 0.108 units [21]. This facilitates the penetration of fosinoprilat through cell membranes and makes it possible to suppress the activity of not only the circulating, but also the tissue RAAS in the heart, lungs, kidneys and brain. It has been experimentally shown that fosinoprilat inhibits ACE in the heart muscle to a greater extent than ramiprilat and enalaprilat [22], which underlies more pronounced (compared to other ACEIs) hypotensive and cardioprotective effects.
Another important property of fosinopril, which has found real application in the clinic, is the double, mutually compensating elimination pathway. Unlike captopril, enalapril, lisinopril and perindopril, which are eliminated from the body primarily by the kidneys, fosinopril is characterized by two main routes of elimination - renal and hepatic (with bile) in a ratio of 1: 1. Moreover, with a decrease in renal function, the excretion of the active metabolite with bile increases and, conversely, with liver failure, its excretion in urine increases. According to special pharmacokinetic studies, in patients with liver cirrhosis, urinary excretion of fosinoprilat increases by 1.5–2 times compared to healthy individuals, and in patients with renal failure, the hepatic excretion pathway increases by 2–3 times. The described feature predetermines the safe use of fosinopril in patients with liver failure (with alcoholic and especially biliary cirrhosis of the liver) and with impaired renal function. Even when the glomerular filtration rate decreases below 15 ml/min, the concentration of fosinopril does not significantly increase, which allows us to consider this drug as a drug of choice in clinical conditions fraught with deterioration of renal function (severe hypertension, concomitant diabetes, metabolic syndrome), and in elderly people with in most cases, concomitant pathology, including diabetic nephropathy. This indicates an important practical advantage of fosinopril over other ACE inhibitors - the absence of the need to monitor renal function when prescribing and increasing dosages, which makes it an optimal drug for outpatient treatment. In addition, no adaptation or dose reduction of fosinopril is required in elderly patients, since no changes in the pharmacokinetics of fosinoprilat were observed in patients 65–74 years of age with clinically normal liver and kidney function compared to young patients (20–35 years of age).
The high safety of fosinopril is of greatest importance.
As is known, the main side effects that limit the use of ACE inhibitors and reduce adherence to treatment, along with deterioration of renal function, are cough and hypotension of the first dose, especially in patients who have suffered an acute MI and have symptoms of CHF. Cough in individuals receiving ACE inhibitors is caused by a blockade of the destruction of bradykinin and some other neurotransmitters in the bronchial mucosa. Its appearance, directly related to the use of ACE inhibitors, usually serves as an indication for replacing ACE inhibitors with a drug from the group of A-II receptor blockers. Fosinopril can also serve as a real alternative in this case. There is evidence that dry cough caused by other ACEIs is reduced or even completely eliminated when switching to fosinopril. For example, a double-blind comparative study with enalapril showed a significantly lower incidence of cough when fosinopril was prescribed. This study included 179 patients who had already stopped taking ACE inhibitors due to the development of cough. An attempt to restart treatment was much more successful when choosing fosinopril - cough recurrence was observed more than half as often as compared with enalapril [20]. Other adverse events are less common when using fosinopril.
Another double-blind study directly compared identical dosages of fosinopril and enalapril (5–20 mg once daily) in the treatment of patients with CHF. Moreover, the number of patients with first-dose hypotension receiving fosinopril was four times less than with enalapril therapy. In addition, when using fosinopril, this study revealed a significantly greater reduction in the risk of the combined endpoint (death + worsening decompensation).
Fosinopril is also distinguished by a convenient dosing regimen - a single dose provides 24-hour blood pressure control (the ratio of residual to peak effect is 64%) and prevents its increase in the early morning hours. The initial daily dose of fosinopril for hypertension is 10 mg once, with a possible subsequent increase to 20–40 mg. For CHF, the initial daily dose is 5-10 mg (in patients with hypotension - 2.5-5 mg), the average therapeutic dose is 10-20 mg, the maximum is 20-40 mg.
Clinical studies of the effectiveness of fosinopril
The high effectiveness of fosinopril in hypertension has been confirmed in international and Russian studies. According to the FOPS study, it effectively reduces blood pressure in 80% of patients, and in patients with mild and moderate hypertension this is not accompanied by compensatory tachycardia. A meta-analysis showed that the antihypertensive activity of fosinopril progressively increases during the first few weeks of treatment until target blood pressure levels are achieved without the manifestation of elements of compensatory heart rhythm disturbances, and drug withdrawal does not cause a rapid rise in blood pressure. The effectiveness of fosinopril is generally independent of age, gender and body weight [23, 24].
In terms of hypotensive activity, fosinopril is not significantly inferior to diuretics, beta-blockers, calcium antagonists and other ACE inhibitors, but is better tolerable and has fewer clinical and biochemical side effects, especially in “risk groups” - in elderly people or patients with diabetes, which was demonstrated in the FACET study, in which the efficacy and safety of fosinopril and the calcium antagonist amlodipine were compared in patients with non-insulin-dependent diabetes and hypertension [25]. Over three years of therapy, with satisfactory and approximately equal blood pressure control, there were significantly fewer deaths, MIs and strokes in the fosinopril group than during amlodipine therapy (14 vs. 27%; p = 0.027). In the FLIGHT study studying the effectiveness of fosinopril in 19,432 patients with hypertension (989 of them over 75 years of age), target blood pressure was achieved in 79.8% of patients 12 weeks after the start of treatment [26].
Within the framework of the Russian FLAG program (Fosinopril in the Treatment of Arterial Hypertension), the probability of achieving target blood pressure levels in patients with mild and moderate hypertension in outpatient settings with monotherapy with fosinopril (10–20 mg/day) or its combination with hydrochlorothiazide was assessed. A total of 2557 patients were included in the study, of which 26.7% were people over 60 years of age. Target blood pressure was achieved in 62.1% of patients. Side effects were noted in 8.3% of patients, and only 5.2% required drug discontinuation [27].
The FAGOT study (Pharmacoeconomic assessment of the use of the ACE inhibitor fosinopril in the outpatient treatment of patients with Complicated Arterial Hypertension) included 2596 patients with mild or moderate hypertension and the presence of two risk factors for cardiovascular complications. The effectiveness of fosinopril monotherapy or its combination with hydrochlorothiazide and conventional therapy (diuretics, beta-blockers, calcium antagonists) was compared in patients of different ages. Target blood pressure when taking fosinopril and hydrochlorothiazide was achieved in 67.8% of patients. It has been shown that the rate of achievement of the hypotensive effect and its severity when using fosinopril do not differ in elderly and young patients, but is higher than when using a traditional treatment regimen. Compared with other drugs, fosinopril was advantageous in its ease of administration and cost-effectiveness [28].
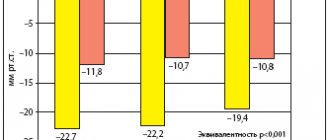
Many studies have proven the effectiveness of fosinopril in CHF. It has been shown that fosinopril not only increases exercise tolerance and reduces FC decompensation (FEST study), but also significantly slows down the progression of CHF. Fosinopril therapy is associated with greater efficacy, safety and a better cost-effectiveness ratio than the use of other ACE inhibitors, in particular enalapril. In the FASON study (Pharmacoeconomic assessment of the use of ACE inhibitors in the outpatient treatment of patients with Heart Failure), which included 1945 patients with FC II–III CHF, in the group of patients with a combination of hypertension and CHF, the decrease in systolic blood pressure was 12.5%, diastolic blood pressure – 11%, and target BP was achieved in 72% of patients [29]. At the same time, treatment costs were reduced by 54%.
Factors that provide the advantage of fosinopril over other ACEIs in patients with CHF include its unique ability to reduce the level of endothelin, a vasoconstrictor neuropeptide that is a predictor of poor prognosis in patients with decompensation [20]. It is possible that this mechanism, along with the known “classical” pathways of action of ACE inhibitors (blockade of A-II synthesis, slowing down the destruction of bradykinin), provides fosinopril with high efficiency when used in patients with acute MI. The randomized, placebo-controlled FAMIS study showed that early (less than 24 hours) addition of fosinopril to thrombolytic therapy in patients with anterior acute myocardial infarction leads to a significant reduction in the incidence of deaths and cases of severe CHF (class III-IV) by 36.2%. [thirty].
As a result of clinical studies, evidence was obtained of the possibility of using fosinopril not only in the treatment of hypertension and CHF, which are related by the common pathogenetic mechanisms of development, but also in the prevention of the progression of cardiac decompensation in patients with a high risk of cardiovascular complications. The high lipophilicity of fosinopril, which allows it to penetrate well into tissues, and the ability to influence the level of vasoconstrictor substances (endothelin) provide maximum organoprotective activity of fosinopril. As a consequence of this, additional effects appear that distinguish this drug from other ACE inhibitors. For example, the PHYLLIS study demonstrated the ability of fosinopril to significantly slow down the development of atherosclerosis of the carotid arteries and prevent an increase in the intima-media ratio of the carotid artery wall in hypertensive patients with asymptomatic atherosclerotic lesions of the carotid system [31]. This opens up prospects for the use of this ACE inhibitor in the treatment of patients with atherosclerosis and to reduce the risk of cerebral stroke in people with hypertension and damage to the carotid arteries.
Of particular interest is the PREVEND–IT study, which proved that, due to its nephroprotective properties, fosinopril can be used as a primary prevention agent in hypertensive patients with microalbuminuria. In addition, it prevents the risk of cardiovascular complications: its use for 4 years in patients with microalbuminuria > 50 mg/24 hours led to a significant reduction in the risk of cerebral stroke and a composite point that included the sum of deaths and hospitalizations due to deterioration of the condition [32 ].
Conclusion
Thus, with the help of fosinopril, one of the most effective and safe representatives of the ACEI class, it is possible not only to successfully treat the most severe patients with hypertension and CHF (elderly, with diabetes, impaired renal function), but also to prevent the development of cardiovascular complications, including including CHF, in persons with multiple risk factors. Therefore, in order to effectively slow down the steady movement of patients along the cardiovascular continuum, it is necessary to prescribe this drug to patients with hypertension as early as possible for the treatment and prevention of progression of CHF. The possibility of using fosinopril to influence diastolic disorders and remodeling as processes underlying the development and progression of CHF in patients with hypertension seems extremely interesting. Despite the existing evidence base and unique pharmacokinetic features, this drug has so far been little studied in this regard, which may determine the prospects for further studies of its effects in patients with a combination of hypertension and CHF.
Side effects of the drug Fozinopril
from the digestive system: rarely - nausea, vomiting, dyspepsia, increased activity of liver transaminases; cases of pancreatitis and hepatitis have been described; from the respiratory system: cough, rarely - pharyngitis, laryngitis, sinusitis, bronchospasm; from the cardiovascular system: palpitations, chest pain, rarely - orthostatic hypotension, collapse, hot flashes, arrhythmia; allergic and immunological reactions - skin rash, itching, photosensitivity, angioedema, myalgia, arthralgia; from the urinary system - proteinuria, oliguria, disorders of excretory function, accompanied by an increase in the concentration of creatinine and urea in the blood plasma; other reactions - dizziness, fatigue, impaired taste and other types of sensitivity.
Fosinopril is an ACE inhibitor with special properties
The classical scheme of the functioning of the RAAS was described in the middle of the twentieth century. (Fig. 1). Renin, formed in the juxtaglomerular apparatus of the kidneys, activates the formation of inactive angiotensin 1 (AT Ι) from angiotensinogen (a peptide produced in the liver), which, under the action of angiotensin-converting enzyme (ACE) (kinase ΙΙ), is converted into active angiotensin ΙΙ (AT ΙΙ). AT ΙΙ binds to two subtypes of receptors: predominantly AT1 and, to a much lesser extent, AT2. Activation of AT1 receptors leads to vasoconstriction, proliferation, activation of the proinflammatory system, dysfunction of the endothelium, and increased secretion of aldosterone. Activation of AT2 receptors causes opposite effects, but in adults their expression is significantly less than AT1 expression [4]. Later, at the end of the twentieth century, it was discovered that all components of the RAAS are autonomously synthesized in the organs and tissues of the human body (myocardium, endothelium, vascular smooth muscle cells, kidneys). It is the activation of tissue RAAS that plays a major role in the development of organ damage in hypertension and diabetes mellitus (DM). A position was formulated on a two-component RAAS, distinguishing circulating and local components that have certain functional differences. The circulating link of the RAAS provides a short-term effect on target organs, and the increase in the activity of the tissue link is sustainable and leads to the formation of not only functional, but also structural changes in organs and tissues. Its activity in tissues remains high, even if the concentration of renin in the blood plasma is normalized (Fig. 2). At the same time, tissue AT II stimulates the growth of fibroblasts, promotes the development of myocardial hypertrophy and hyperplasia, remodeling of the heart and blood vessels, and participates in cellular apoptosis reactions. Thus, an increase in the activity of the RAAS, both circulating in the blood and tissue, is one of the most important pathogenetic mechanisms for the formation of hypertension, target organ damage in cardiovascular diseases and diabetes, and the development of chronic heart failure (CHF). ACE inhibitors (ACEIs) are a group of drugs that reduce the activity of the RAAS. The first ACE inhibitor, teprotide, was obtained in 1971 from the venom of the Brazilian snake Jararaca (Fig. 3). But due to its toxicity, this drug was almost never used in clinical practice. In 1975, D. Cushman and M. Ondetti (Fig. 4) synthesized the first oral ACE inhibitor, captopril, which became widely used in clinical practice. The era of ACE inhibitors has begun. Currently, there are more than 10 drugs of the ACE inhibitor group, differing from each other in chemical structure and pharmacokinetic parameters. According to the classification proposed in 1994 by L. Opie [5], all ACE inhibitors are divided into 3 classes and subgroups (Table 1). Lipophilic (capable of soluble in fat) ACE inhibitors (class I) are usually protein bound, have pharmacological activity, and are metabolized in the liver to form active and inactive metabolites, which are excreted by the kidneys and penetrate the blood-brain barrier. Class II are prodrugs that, after hydrolysis in the liver, become active forms. Class III drugs are not metabolized in the body, circulate in the blood without binding to plasma proteins and are excreted unchanged by the kidneys. Hydrophilic ACE inhibitors bind little to proteins, which reduces their bioavailability. In addition, ACE inhibitors are divided according to their duration of action into: – short-acting ACE inhibitors, which must be prescribed 2 or 3 times a day (for example, captopril); – ACE inhibitors with an average duration of action, which must be taken at least 2 times a day (zofenopril and enalapril); – long-acting ACE inhibitors, they are taken 1 time per day (quinapril, lisinopril, perindopril, ramipril, spirapril, trandolapril, fosinopril). Depending on the chemical structure, ACE inhibitors are distinguished containing: – a sulfhydryl group (captopril, zofenopril, etc.); – carboxyalkyl group (enalapril, lisinopril, perindopril, ramipril, etc.); – phosphinyl group (fosinopril). Moreover, the presence of the SH group is associated with the development of such undesirable effects as hematotoxicity, taste disturbance, and headache. At the end of the twentieth century. a group of researchers led by D. Cushman synthesized fosinopril, which differs in its chemical structure from other ACE inhibitors. Fosinopril and its active metabolite fosinoprilat are proline derivatives of phosphinic acid. The phosphinyl group binds to the zinc ion in the active site of ACE, inhibiting its activity. Fosinopril is a prodrug and only after absorption in the small intestine and hydrolysis in the liver does it become the active metabolite - fosinoprilate. A special feature of fosinopril is that its metabolism occurs not only in the liver, but also in other organs (the mucous membrane of the gastrointestinal tract, kidneys, blood vessels). The half-life of the drug is from 12 to 15 hours, which allows it to be prescribed once a day. With moderate renal impairment, the half-life increases to 14–32 hours, but no significant accumulation of fosinopril is observed. The high lipophilicity of fosinopril determines its wide distribution in the body, and therefore its ability to inhibit the activity of not only ACE circulating in the blood, but also the tissue part of the RAAS (in the heart, lungs, kidneys, brain). The experiment showed that fosinopril suppresses ACE activity in the myocardium to a greater extent than enalapril, lisinopril and ramipril. The main pharmacokinetic parameters of fosinopril do not differ significantly between young and elderly patients. Since the drug has 2 main routes of elimination (through the kidneys and bile), no dose adjustment is required for moderate renal/liver dysfunction [6–8]. The starting dose of fosinopril is 10 mg/day, incl. in elderly patients and with mild renal and liver dysfunction. If the effect is insufficient, the dose can be increased to 20–40 mg/day under blood pressure control [9]. Due to its unique pharmacodynamic and pharmacokinetic parameters, fosinopril is widely used in the treatment of patients with hypertension and CHF, incl. with post-infarction cardiosclerosis, diabetes and diastolic myocardial dysfunction. The results of controlled clinical studies showed that fosinopril at a dose of 10–40 mg/day reduces blood pressure by an average of 14–29/7–18 mm Hg. Art. According to controlled studies, fosinopril monotherapy is effective in the majority of patients with non-severe hypertension (from 50 to 92%), regardless of race, gender and with isolated systolic hypertension in the elderly [11]. With a single dose, fosinopril effectively reduces blood pressure throughout the day, incl. and in the early morning hours [12]. Adverse events with fosinopril are rare and, according to controlled clinical studies, are comparable to those in the placebo group. When taking fosinopril, “first dose hypotension” does not develop, which makes it the drug of choice in the treatment of patients with CHF receiving diuretics. The drug does not significantly affect aldosterone levels, and therefore, hyperkalemia occurs less frequently when taking it. Cough when taking fosinopril is much less common than when treated with other ACE inhibitors (Fig. 5) [10]. Taking fosinopril, like all ACEIs, is contraindicated in pregnant women, in the presence of stenosis of both renal arteries and hypersensitivity reactions. The use of fosinopril in patients with hypertension significantly reduces the incidence of cardiovascular events and death from all causes compared to amlodipine (Fig. 6). During long-term (12 weeks) administration of fosinopril, a significant decrease in left ventricular myocardial hypertrophy was observed compared with the placebo group (Fig. 7). When compared with captopril and lisinopril, fosinopril inhibits myocardial ACE to a significantly greater extent, which leads to a more pronounced improvement in both systolic and diastolic functions of the left ventricle, an increase in stroke and systolic volumes, and maximum ejection rate. During therapy with fosinopril, when blood pressure was stabilized at the target level, not only was there no decrease in renal blood flow and renal filtration; on the contrary, with prolonged use, these indicators increase. At the same time, a decrease in the level of proteinuria is observed. It is important that these favorable changes are also observed in the treatment of a particularly prognostically unfavorable category of patients - when hypertension is combined with diabetes. Fosinopril, like other ACE inhibitors, does not have a negative effect on blood glucose levels [13, 14]. There is evidence of a decrease in the expression of Toll receptors under the influence of fosinopril in patients with hypertension and impaired renal function. This may indicate an anti-inflammatory effect of the drug, which is accompanied by a decrease in endothelial dysfunction and an additional nephroprotective effect [15]. The combination of fosinopril with calcium antagonists (amlodipine), diuretics (hydrochlorothiazide, indapamide) potentiates the hypotensive effect and is well tolerated with long-term therapy [16, 17]. In patients with CHF during therapy with fosinopril, positive clinical dynamics are observed, increased tolerance to physical activity, the need for hospital treatment decreases, and indicators of myocardial contractile function improve. A decrease in patient mortality was noted. Moreover, the effectiveness of treatment with fosinopril is higher than with other ACE inhibitors, in particular enalapril (Fig. 8). The use of fosinopril leads to a decrease in pulmonary artery pressure. Thus, in a study by C. Martiniuc, A. Braniste, T. Braniste (2012), after 2 months of taking the drug, a decrease in mean and systolic pressure in the pulmonary artery was observed [19]. Thus, the long-acting ACE inhibitor fosinopril, due to its chemical structure, is capable of inhibiting both the circulating and tissue RAAS. Fosinopril not only effectively lowers blood pressure, but also has a clinically significant organoprotective effect, which makes it the drug of choice in the treatment of patients with hypertension and diabetes. Fosinopril improves the clinical course of CHF and increases the survival of these patients. Fosinopril therapy is well tolerated. The drug can be used in patients of older age groups, with mild renal and liver dysfunction.
Literature 1. Mareev V.Yu. Blockade of the renin-angiotensin-aldosterone system at different levels // Practitioner. 2000. No. 18. pp. 23–24. 2. Schrier RW, Abraham WT Mechanisms of disease: Hormones and hemodynamics in heart failure // New Engl. J. Med. 1999. Vol. 341. No. 8. P. 577–585. 3. Sidorenko B.A., Preobrazhensky D.V. Diagnosis and treatment of arterial hypertension. Part 3. ACE inhibitors and AT1-angiotensin receptor blockers. M.: Persid, 2001. 164 p. 4. Dihn DT, Frauman AG, Jonston CI, Fabiani CI Angiotensin receptors: distribution, signaling and function // Clinical Sci. 2001. Vol. 100. P. 481–492. 5. Opie LH Angiotensin converting enzyme inhibitors. 3rd edition. NY: Wiley-Liss, 1999. 6. Sidorenko B.A., Preobrazhensky D.V., Batyraliev T.A. ACE inhibitors and AT1-angiotensin blockers in clinical practice of the converting enzyme. Part 3. M.: JSC Presid-Alliance, 2004. 7. Guthrie R. Fosinopril: an overview // Am J Cardiol. 1993. Vol. 72. R. 22–24. 8. Grover GJ, Sleph PG, Dzwonczyk S. et al. Effects of different angiotensin-converting enzyme (ACE) inhibitors on ischemic isolated rat hearts: relationship between cardiac ACE inhibition and cardioprotection // J Pharmacol Exp Ther. 1991. Vol. 257. R. 919–929. 9. Federal guidelines for the use of drugs. M., 2013. 10. Martin LC, Velasco-Cornejro IF, Franco RJ Treatment of mild and moderate hypertension with fosinopril. Comparison of adverse effects with other antihypertensive agents // Arq Bras Cardiol. 1994. Vol. 62(5). R. 369–374. 11. Pool JL Antihypertensive effect of fosinopril, a new angiotensin-converting enzyme inhibitor: Findings on the Fosinopril Study Group II // Clin Ther. 1990. Vol. 12. R. 520–527. 12. Anderson RJ, Duchin KL, Gore RD et al. Once-daily fosinopril in the treatment of hypertension // Hypertension. 1991. Vol. 17. R. 636–642. 13. Tatti P., Guarisco R., Pahor M. et al. Outcome results of the Fosinopril Versus Amlodipine Cardiovascular Events Randomized Trial (FACET) in patients with hypertension and NIDDM // Diabetes Care. 1998. Vol. 21(4). R. 597–603. 14. Cheung BM, Lau CP Fosinopril reduces left ventricular mass in untreated hypertensive patients: a controlled trial // Br J Clin Pharmacol. 1999 Feb. Vol. 47(2). R. 179–187. 15. Tang TF, Zhou QL, Zhu LL, Tang R., Ao X. Effects of fosinopril and losartan on the expression of Toll-like receptor 4 in renal tubular epithelia cells // Zhong Nan Da, Xue Xue Bao, Yi Xue Ban . 2008 Oct. Vol. 33 (10). R. 958–965. 16. Meng Y., Zhang Z., Liang X., Wu C., Qi G. Effects of combination therapy with amlodipine and fosinopril administered at different times on blood pressure and circadian blood pressure pattern in patients with essential hypertension // Acta Cardiol . 2010 Jun. Vol. 65(3). R. 309–314. 17. Zhang JL, Qin YW, Zheng X., Qiu JL, Zhao XX, Zou DJ Combination therapy with angiotensin-converting enzyme inhibitors and indapamide impairs glucose tolerance in Chinese hypertensive patients // Blood Press. 2010 Apr. Vol. 19 (2).110-8. 18. Blumenthal M. Treatment of congestive heart failure: experience with fosinopril // Am J Hypertens. 1997 Oct. Vol. 10 (10 Pt 2). R. 289–298. 19. Martiniuc C., Braniste A., Braniste T. Angiotensin converting enzyme inhibitors and pulmonary hypertension // Rev Med Chir Soc Med Nat Iasi. 2012 Oct-Dec.Vol. 116(4). R. 1016–1020.
Special instructions for the use of the drug Fozinopril
Prescribe with caution to patients with renovascular hypertension, heart failure, patients on hemodialysis, as well as patients with hypovolemia and/or reduced plasma osmolarity of any etiology due to the increased risk of developing side effects from the kidneys. In order to reduce the risk of arterial hypotension, diuretics should be discontinued 2-3 days before fosinopril is prescribed and the water and electrolyte balance should be corrected. In patients with left ventricular hypertrophy, long-term use of fosinopril leads to a decrease in left ventricular mass and a decrease in the thickness of the interventricular septum. After discontinuation of fosinopril, no withdrawal syndrome (sharp increase in blood pressure) is observed. The safety and effectiveness of fosinopril in pediatric practice has not been established. No special adjustment of the fosinopril dosage regimen is required in elderly patients. Women of reproductive age receiving fosinopril should use reliable contraception.
Fosinopril, 5 mg, tablets, 28 pcs.
2-3 days before starting treatment with fosinopril, it is recommended to discontinue previous diuretic therapy, with the exception of patients with malignant or difficult-to-treat hypertension. In such cases, fosinopril therapy should be started immediately, at a reduced dose, with close medical supervision and careful dose escalation.
Symptomatic hypotension with the use of ACE inhibitors most often develops in patients after intensive treatment with diuretics, a diet limiting salt intake, or during renal dialysis. Transient arterial hypotension is not a contraindication for continuing treatment after measures to restore blood volume.
In patients with chronic heart failure, treatment with ACE inhibitors may cause excessive antihypertensive effects, which can lead to oliguria or azotemia, which can be fatal. Therefore, when treating patients with chronic heart failure with fosinopril, careful clinical monitoring is necessary, especially during the first 2 weeks of treatment, as well as with any increase in the dose of fosinopril or diuretic.
ACE inhibitors rarely cause swelling of the intestinal mucosa. In this case, patients experience abdominal pain (sometimes without nausea and vomiting), facial swelling may also be absent, and the level of C1-esterases is normal. After stopping taking ACE inhibitors, symptoms disappear. Swelling of the intestinal mucosa should be taken into account in the differential diagnosis of patients with abdominal pain while taking ACE inhibitors.
During treatment with ACE inhibitors during hemodialysis using highly permeable membranes, as well as during LDL apheresis with adsorption on dextran sulfate, anaphylactic reactions may develop. In these cases, the use of a different type of dialysis membrane or other antihypertensive therapy should be considered.
It is possible to develop agranulocytosis and suppression of bone marrow function during treatment with ACE inhibitors. These cases occur more often in patients with impaired renal function, especially in the presence of systemic connective tissue diseases (systemic lupus erythematosus or scleroderma). Before starting therapy with ACE inhibitors and during treatment, the total number of leukocytes and the leukocyte formula are determined (once a month in the first 3-6 months of treatment and in the first year of treatment in patients with an increased risk of neutropenia).
If noticeable jaundice and a marked increase in liver enzyme activity occur, fosinopril treatment should be discontinued and appropriate treatment should be prescribed.
In case of arterial hypertension in patients with bilateral renal artery stenosis or stenosis of the artery of a single kidney, as well as with the simultaneous use of diuretics without signs of renal impairment during treatment with ACE inhibitors, the concentration of blood urea nitrogen and serum creatinine may increase. These effects are usually reversible and disappear after treatment is stopped. A dose reduction of diuretic and/or fosinopril may be required.
In patients with severe chronic heart failure with altered RAAS activity, treatment with ACE inhibitors can lead to oliguria, progressive azotemia and, in rare cases, acute renal failure and possible death.
During fosinopril therapy, the patient should be careful when performing physical exercise or in hot weather due to the risk of dehydration and hypotension due to a decrease in blood volume.
No special adjustment of the fosinopril dosage regimen is required in elderly patients. Safety of use in children has not been established.
Before and during treatment with the drug, it is necessary to monitor blood pressure, kidney function, potassium levels, hemoglobin levels, creatinine, urea, electrolyte concentrations and the activity of liver transaminases in the blood.
Influence on the ability to drive vehicles and operate machinery.
Caution is required when driving vehicles or performing other work that requires increased attention, because Dizziness may occur, especially after the initial dose of fosinopril.
Fosinopril-OBL (Fosinopril-OBL)
Arterial hypotension
In patients with uncomplicated arterial hypertension, arterial hypotension may develop due to the use of fosinopril. Symptomatic arterial hypotension when using ACE inhibitors more often develops in patients during intensive treatment with diuretics, a diet associated with limiting sodium chloride, or during dialysis. Transient arterial hypotension is not a contraindication for the use of fosinopril after measures to restore blood volume.
In patients with chronic heart failure, treatment with ACE inhibitors may cause excessive antihypertensive effects, which can lead to oliguria or azotemia and, in rare cases, fatal acute renal failure. Therefore, when treating chronic heart failure with fosinopril, patients should be closely monitored, especially during the first 2 weeks of treatment, as well as with any increase in the dose of fosinopril or diuretic.
It may be necessary to reduce the diuretic dose in patients with normal or low blood pressure, who have previously received diuretic therapy, or who have hyponatremia. Arterial hypotension as such is not a contraindication for further use of fosinopril in chronic heart failure. Some reduction in systemic blood pressure is a common and desirable effect when initiated in chronic heart failure. The extent of this reduction is greatest early in treatment and stabilizes within one or two weeks of starting treatment. Blood pressure usually returns to baseline levels without a decrease in therapeutic efficacy.
Before starting treatment, it is necessary to analyze previous antihypertensive therapy, the degree of increase in blood pressure, dietary restrictions on salt and/or liquid, and other clinical circumstances. If possible, previous antihypertensive therapy should be discontinued several days before starting treatment. To reduce the likelihood of arterial hypotension, diuretics should be discontinued 2-3 days before starting treatment. Before and during treatment, it is necessary to monitor blood pressure, renal function, the content of potassium ions, creatinine, urea, electrolyte concentrations and the activity of liver enzymes in the blood.
Aortic or mitral stenosis/hypertrophic obstructive cardiomyopathy As with all drugs that have a vasodilating effect, ACE inhibitors should be used with extreme caution in patients with left ventricular outflow tract obstruction.
Renal dysfunction
In patients with arterial hypertension with unilateral or bilateral renal artery stenosis or stenosis of the artery of a solitary kidney, the concentration of blood urea nitrogen and serum creatinine may increase during treatment with ACE inhibitors. These effects are usually reversible and disappear after treatment is stopped. It is necessary to monitor renal function in such patients in the first weeks of treatment. In some patients, increases in blood urea nitrogen and serum creatinine concentrations (usually small and transient) may be observed even without obvious renal impairment when fosinopril and diuretics are used concomitantly. A dose reduction of fosinopril may be required.
In patients with severe chronic heart failure, renal function may be dependent on the activity of the renin-angiotensin-aldosterone system, so treatment with ACE inhibitors may be accompanied by oliguria and/or progressive azotemia, and in rare cases may lead to acute renal failure and death.
Kidney transplant
There is no experience with the use of fosinopril in patients who have recently undergone kidney transplantation.
Liver dysfunction
In rare cases, when using ACE inhibitors, a syndrome is observed, the first manifestation of which is cholestatic jaundice. This is followed by fulminant liver necrosis, sometimes fatal. The mechanism of development of this syndrome has not been studied. If noticeable icterus and a marked increase in liver enzyme activity occur, fosinopril treatment should be discontinued and appropriate treatment should be prescribed.
In patients with impaired liver function, increased plasma concentrations of fosinopril may be observed. In liver cirrhosis (including alcoholic cirrhosis), the apparent total clearance of fosinoprilat is reduced, and the AUC is approximately 2 times higher than in patients without liver dysfunction.
Neutropenia/agranulocytosis/thrombocytopenia/anemia
It is possible to develop agranulocytosis and suppression of bone marrow function during treatment with ACE inhibitors. These cases occur more often in patients with impaired renal function, especially in the presence of systemic connective tissue diseases (SLE or scleroderma). Before starting therapy with ACE inhibitors and during treatment, leukocytes and leukocyte formula are determined (once a month in the first 3-6 months of treatment and in the first year of fosinopril use in patients with an increased risk of neutropenia).
Hypersensitivity reactions/angioedema
The development of angioedema of the extremities, face, lips, mucous membranes, tongue, pharynx or larynx has been reported in patients receiving fosinopril. Swelling of the tongue, pharynx, or larynx can cause airway obstruction, which can be fatal. In such cases, it is necessary to stop taking fosinopril and take emergency measures, including subcutaneous injection of epinephrine (adrenaline) solution (1:1000), as well as other emergency treatment measures. In most cases of swelling of the face, oral mucosa, lips and extremities, stopping fosinopril resulted in normalization of the condition; however, appropriate therapy was sometimes required.
Swelling of the intestinal mucosa
Swelling of the intestinal mucosa has rarely been observed while taking ACE inhibitors. Patients complained of abdominal pain (there may have been no nausea and vomiting); in some cases, swelling of the intestinal mucosa occurred without swelling of the face; C1-esterase activity was normal. Symptoms disappeared after stopping the use of ACE inhibitors. Edema of the intestinal mucosa should be included in the differential diagnosis of patients taking ACE inhibitors who complain of abdominal pain.
Patients with a history of angioedema not associated with ACE inhibitors may be at greater risk of developing angioedema during ACE inhibitor therapy.
In representatives of the Negroid race, cases of the development of angioedema when using ACE inhibitors were observed with a higher frequency compared to representatives of other races.
An increased risk of angioedema was observed in patients concomitantly taking ACE inhibitors and drugs such as mTOR inhibitors (temsirolimus, sirolimus, everolimus), dipeptidyl peptidase type IV inhibitors (sitagliptin, saxagliptin, vildagliptin, linagliptin), estramustine, neutral endopeptidase inhibitors (racecadotril). , sacubitril) and tissue plasminogen activators.
Anaphylactic reactions during desensitization
In two patients, during desensitization with hymenoptera venom while taking the ACE inhibitor enalapril, life-threatening anaphylactoid reactions were noted. In the same patients, these reactions were avoided by timely interruption of the ACE inhibitor; however, they reappeared after inadvertent resumption of an ACE inhibitor. Particular care should be taken when desensitizing patients taking ACE inhibitors.
Anaphylactoid reactions during low-density lipoprotein apheresis (LDL apheresis)
Life-threatening anaphylactoid reactions have rarely been observed in patients taking ACE inhibitors during LDL apheresis using dextran sulfate. The development of these reactions can be prevented by temporarily discontinuing the ACE inhibitor before each LDL apheresis procedure.
Hemodialysis using high-flow membranes
When performing hemodialysis in patients receiving ACE inhibitors, the use of high-flow polyacrylonitrile dialysis membranes (for example, AN69) should be avoided, since in such cases the risk of developing anaphylactoid reactions increases. In such cases, it is necessary to use a different type of dialysis membrane or use antihypertensive drugs of other classes.
Cough
When using ACE inhibitors, including fosinopril, a non-productive, persistent cough was observed, which disappeared after discontinuation of therapy. When cough occurs in patients taking ACE inhibitors, this therapy should be considered as a possible cause in the differential diagnosis.
Surgery/general anesthesia
ACE inhibitors may enhance the antihypertensive effect of drugs used for general anesthesia. Before surgery (including dentistry), you must warn your doctor/anesthesiologist about the use of ACE inhibitors. Caution should be exercised when performing physical exercise or in hot weather due to the risk of dehydration and hypotension due to a decrease in blood volume.
Hyperkalemia
There have been cases of increased levels of potassium ions in the blood serum of patients taking ACE inhibitors, incl. fosinopril At risk in this regard are patients with renal failure, type 1 diabetes mellitus, as well as those taking potassium-sparing diuretics (such as spironolactone, eplerenone, triamterene or amiloride), potassium supplements, potassium-containing nutritional supplements or other drugs that increase the level of potassium ions in the serum blood. If necessary, simultaneous use of fosinopril and the above potassium-containing or increasing potassium levels in the blood plasma drugs should be used with caution and regularly monitor the potassium level in the blood serum.
Dual blockade of the renin-angiotensin-aldosterone system (RAAS)
The simultaneous use of drugs from different groups that act on the RAAS is not recommended (double blockade of the RAAS), since it has been associated with an increased incidence of side effects such as arterial hypotension, hyperkalemia, and decreased renal function (including acute renal failure).
The simultaneous use of ACE inhibitors with drugs containing aliskiren is contraindicated in patients with diabetes mellitus and/or with moderate or severe renal impairment (GFR less than 60 ml/min/1.73 m2 body surface area) and is not recommended in other patients.
Concomitant use of ACE inhibitors with angiotensin II receptor antagonists is contraindicated in patients with diabetic nephropathy and is not recommended in other patients.
Ethnic differences
ACE inhibitors are less effective in blacks than in Caucasians, which may be due to the higher prevalence of low renin activity in blacks.