1.General information
The term “acidosis” can be translated from Latin as “total acidification.” This is the name given to the decrease in the total hydrogen index (pH) in the body relative to its standard, natural values: 7.37 - 7.44. Maintaining the acid-base balance within these limits is one of the most important elements of homeostasis (constancy of internal conditions), since its strictly defined and fairly narrow range is necessary for the effective occurrence of all biochemical and neurotransmitter reactions, digestive processes, the functioning of symbiotic microflora, and many others.
Acidosis accompanies many diseases, pathological processes and conditions in which the circulation and removal of organic acids from the body is disrupted. The range of such conditions is very wide - from pregnancy and long-term malnutrition to severe infections and endocrine-metabolic disorders (for example, diabetes mellitus). Thus, acidosis is not an independent disease; This is a secondary syndrome that develops due to certain pathogenetic patterns.
A must read! Help with treatment and hospitalization!
Treatment of lactic acidosis
- Hemodialysis - infusion therapy is used to remove accumulated lactate from the cardiovascular system. Intravenous glucose and insulin injections trigger enzyme and protein activity.
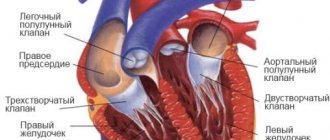
- Artificial pulmonary ventilation (ALV) with intubation of the patient restores gas exchange, maintains impaired respiratory function, and reduces the concentration of carbon dioxide in the blood to normal levels. The procedure is carried out until the alkaline balance is restored.
- Cardiotonic drugs are prescribed to stimulate the activity of the heart muscle and normalize heart rhythm.
- Insulin therapy with prolonged effect.
As an emergency aid, the patient is administered sodium bicarbonate with a concentration of 4% or 2.5%, the daily dose is up to 2 liters. The drug is administered as a slow intravenous infusion, with adequate ventilation and calcium supplementation to mitigate side effects.
For patients with septic shock, crystalloid solutions - saline or saline - are prescribed in an amount of 30 mg/kg for the first 3 hours. Broad-spectrum antibiotics are used to combat concomitant infections.
Metformin
In case of lactic acidosis, the prescription of metformin to patients with type 2 diabetes is associated with the risk of complications due to possible overdose.
If metabolic acidosis is suspected, metformin should be stopped immediately and the patient should be hospitalized. It is important to preserve the vital functions of the body by stabilizing blood circulation and ensuring complete saturation of blood and tissues with oxygen. There is no specific antidote for metformin. In case of overdose, the introduction of activated carbon for adsorption may be indicated after a few hours.
2. Reasons
The main reasons for the shift in acid-base balance towards acidification include:
- smoking, drinking alcohol;
- malnutrition (regardless of reasons);
- insufficient fluid intake and dehydration (dehydration) of any other origin;
- intoxication with impaired digestive function of the gastrointestinal tract;
- carrying a pregnancy;
- metabolic disorders;
- cancer;
- hypoxia of any origin;
- a number of kidney diseases.
Visit our Nephrology page
Biochemistry of exercise-induced metabolic acidosis
Robergs, Robert A., Farzenah Ghiasvand, and Daryl Parker. Biochemistry of exercise-induced metabolic acidosis. Am J Physiol Regul Integr Comp Physiol. 287: R502–R516, 2004; 10.1152/ajpregu.00114.2004
annotation
The development of acidosis (muscle acidification) during intense exercise is traditionally explained by increased production of lactic acid, which releases a hydrogen proton and produces an acidic salt, sodium lactate. Based on this explanation, if the level of lactate production is high enough, the buffering capacity of the cells may be exceeded, resulting in decreased cellular (i.e., muscle acidification). These biochemical events are called lactic acidosis.
Lactic acidosis during exercise has been the classic explanation for the biochemistry of muscle acidosis (acidification of the mouse) for over 80 years. This belief has led to the interpretation that lactate production causes muscle acidosis and, in turn, that increased lactate production is one of several causes of muscle fatigue during intense exercise.
This review shows that there is no biochemical evidence that lactate production causes acidosis.
The cause of muscle acidosis (muscle acidification) is a slowdown in lactate production . Likewise, there are many studies showing that acidosis is caused by reactions other than lactate production. Each time ATP breaks down into ADP and inorganic phosphate, a hydrogen proton is released. When the need for ATP during muscle contraction is based on mitochondrial respiration (tissue respiration), hydrogen protons do not accumulate in muscle fibers, since protons are used by mitochondria for oxidative phosphorylation and to maintain the proton gradient in the intermembrane space. Proton accumulation occurs only when exercise intensity is increased beyond steady state, which occurs to a greater extent during ATP resynthesis based on glycolysis and the phosphagen system. ATP, which comes from these non-mitochondrial sources, is ultimately used as fuel to reduce muscle nutrition. increases proton release and causes acidosis during intense exercise.
In this case, lactate production is increased to prevent the accumulation of pyruvate and supply NAD+, necessary for the second phase of glycolysis. Thus, increased lactate production coincides with cellular acidosis and remains a good indirect marker of the cellular metabolic conditions causing metabolic acidosis. If muscles do not produce lactate, acidosis occurs more often and muscle fatigue occurs more quickly, and exercise performance will be seriously impaired.
Keywords
skeletal muscle, lactate, acidosis
The structure and functions of muscles are described in more detail in my books:
- Human skeletal muscle hypertrophy
- Biomechanics of the human musculoskeletal system
Abstract
The development of acidosis during intense exercise has traditionally been explained by the increased production of lactic acid, causing the release of a proton and the formation of the acid salt sodium lactate. On the basis of this explanation, if the rate of lactate production is high enough, the cellular proton buffering capacity can be exceeded, resulting in a decrease in cellular pH. These biochemical events have been termed lactic acidosis.
The lactic acidosis of exercise has been a classic explanation of the biochemistry of acidosis for more than 80 years. This belief has led to the interpretation that lactate production causes acidosis and, in turn, that increased lactate production is one of the several causes of muscle fatigue during intense exercise.
This review presents clear evidence that there is no biochemical support for lactate production causing acidosis. Lactate production retards, not causes, acidosis.
Similarly, there is a wealth of research evidence to show that acidosis is caused by reactions other than lactate production. Every time ATP is broken down to ADP and Pi, a proton is released. When the ATP demand of muscle contraction is met by mitochondrial respiration, there is no proton accumulation in the cell, as protons are used by the mitochondria for oxidative phosphorylation and to maintain the proton gradient in the intermembranous space. It is only when the exercise intensity increases beyond steady state that there is a need for greater reliance on ATP regeneration from glycolysis and the phosphagen system.
The ATP that is supplied from these nonmitochondrial sources and is eventually used to fuel muscle contraction increases proton release and causes the acidosis of intense exercise. Lactate production increases under these cellular conditions to prevent pyruvate accumulation and supply the NAD_ needed for phase 2 of glycolysis. increased lactate production coincides with cellular acidosis and remains a good indirect marker for cell metabolic conditions that induce Thus metabolic acidosis. If muscle did not produce lactate, acidosis and muscle fatigue would occur more quickly and exercise performance would be severely impaired.
3. Symptoms and diagnosis
Since acidosis occurs against the background and as a result of some pathology, and with its significant severity or long-term course, in most cases the clinical picture of the underlying disease (condition) dominates.
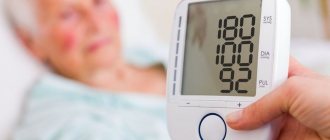
In mild forms, acidosis can manifest itself as weakness, fatigue, nausea, and vomiting. In more severe cases, the so-called Kussmaul breathing (deep, noisy), blood pressure rises, heart rhythm is disrupted. If the pathological “vicious circle” causing acidosis is not interrupted at this stage, then very serious depression of the central nervous system may occur (up to confusion and loss of consciousness) or hypovolemic shock may develop (a life-threatening condition caused by a sudden rapid reduction in the volume of circulating blood and, accordingly, deficiency of tissue oxygenation). A further shift in pH to the acidic side (to 7.1 - 7.2 and below) inevitably leads to coma and then death.
Since the symptoms described above are mainly nonspecific (even in aggregate), acidosis and the degree of its severity are established by laboratory tests of blood and urine (clinical analysis, analysis of gas composition, serum analysis of electrolytes, etc.). It is much more important to identify not the acidosis itself, but its causes, and this must be done as soon as possible, since with some diseases leading to acidosis the patient’s condition can worsen to critical very quickly.
About our clinic Chistye Prudy metro station Medintercom page!
Symptoms of lactic acidosis
The first signs of pathology are dyspeptic disorders, muscle pain, attacks of sudden pain in the chest, complete ineffectiveness of painkillers. The patient develops disturbances of consciousness. This condition sometimes resembles alcohol intoxication.
With lactic acidosis, abdominal pain often occurs, vomiting, diarrhea, tachypnea and hypothermia are also characteristic. Cardiovascular arrhythmias, hypotension and decreased cardiac output are possible. The neurological picture can be equally varied: from mild confusion, asthenia to deep coma.
If the patient's condition worsens sharply, it is necessary to act immediately:
- Call an ambulance;
- If the patient is breathing and the pulse is palpable, it is necessary to provide him with access to fresh air and place him on his left side;
- If a person is unconscious, the pulse cannot be felt, there is no breathing - proceed to chest compressions;
- If a person is unconscious, do not try to give him something to drink.
Non-gas acidosis
Non-gas acidosis develops through three main mechanisms:
- excessive formation of acidic metabolites in the body, their accumulation and shift in blood pH;
- violation of the excretion of metabolites (they are formed in normal quantities, but cannot leave the body, so they accumulate in it);
- the entry from outside of substances that shift the pH of the blood in quantities exceeding the capabilities of the body’s excretory system.
The first mechanism is most often activated in diabetes mellitus. Against the background of impaired glucose metabolism, lactic acid and ketone bodies accumulate in the body.
Impaired elimination is most often associated with renal failure or hyperaldosteronism: the kidneys lose the ability to remove excess hydrogen ions from the blood.
The introduction of certain drugs into the body can result in acidosis. It is caused by severely exceeding the dose of medication (for example, a suicide attempt) or taking medications against the background of impaired function of the excretory system. Acidosis is often provoked by acetylsalicylic acid taken for a long time in high doses.