Lisinopril
Arterial hypotension
Most often, a pronounced decrease in blood pressure occurs with a decrease in blood volume caused by diuretic therapy, a decrease in the content of sodium chloride in food, dialysis, diarrhea or vomiting. In patients with CHF and with or without concurrent renal failure, a pronounced decrease in blood pressure is possible. When using the drug Lisinopril, some patients with CHF, but with normal or reduced blood pressure, may experience a marked decrease in blood pressure, which is usually not a reason to discontinue treatment.
In such patients, treatment with Lisinopril should be started under the strict supervision of a physician (with caution in selecting the dose of the drug and diuretics).
Before starting treatment with the drug, if possible, the sodium content should be normalized and/or the blood volume should be replenished, and the effect of the initial dose of Lisinopril on the patient should be carefully monitored.
Under strict medical supervision, Lisinopril should be used in patients with coronary heart disease, cerebrovascular insufficiency, in whom a sharp decrease in blood pressure can lead to myocardial infarction or stroke. Transient arterial hypotension is not a contraindication for further use of the drug. After blood pressure has been restored, it is possible to continue using the drug.
Renal dysfunction
In patients with impaired renal function (creatinine clearance less than 80 ml/min), the initial dose of lisinopril should be changed in accordance with the creatinine clearance (see section "Dosage and Administration"). Regular monitoring of potassium levels and creatinine concentrations in blood plasma is a mandatory treatment strategy for such patients. In patients with CHF, arterial hypotension can lead to deterioration of renal function. Cases of acute renal failure, usually reversible, have been reported in such patients. In case of acute myocardial infarction, drug therapy should not be started in patients with signs of renal impairment, i.e. with a plasma creatinine concentration of 177 µmol/l and/or proteinuria more than 500 mg/day. If renal function is impaired during the use of the drug (creatinine concentration exceeds 265 µmol/l or its value doubles before the start of therapy), it is necessary to consider discontinuing the drug.
Renal artery stenosis
In case of renal artery stenosis (especially with bilateral stenosis or in the presence of stenosis of the artery of a single kidney), as well as with peripheral circulatory failure due to a lack of sodium ions and/or fluid, the use of the drug Lisinopril can lead to impaired renal function, acute renal failure, which usually turns out to be irreversible even after discontinuation of the drug.
In acute myocardial infarction
The drug Lisinopril can be used simultaneously with standard therapy for acute myocardial infarction (thrombolytics, acetylsalicylic acid as an antiplatelet agent, beta-blockers).
The drug Lisinopril can be used simultaneously with a solution of nitroglycerin for intravenous administration or with nitroglycerin for administration using therapeutic transdermal systems, as well as nitroglycerin for sublingual use. The use of Lisinopril is not recommended in patients who have suffered acute myocardial infarction if systolic blood pressure does not exceed 100 mmHg. rt. Art. In case of persistent arterial hypotension (SBP less than 90 mm Hg for more than 1 hour), the drug must be discontinued.
Surgery/general anesthesia
During extensive surgical interventions, as well as when using other drugs that cause a decrease in blood pressure, lisinopril, by blocking the formation of angiotensin II, can cause a pronounced, unpredictable decrease in blood pressure. Before surgery (including dentistry), the surgeon/anesthesiologist should be informed about the use of an ACE inhibitor.
In elderly patients, the use of standard doses leads to higher concentrations of lisinopril in the blood, so special care is required when determining the dose.
Mitral stenosis/aortic stenosis/hypertrophic obstructive cardiomyopathy
Lisinopril, like other ACE inhibitors, should be administered with caution to patients with left ventricular outflow tract obstruction (aortic stenosis, hypertrophic obstructive cardiomyopathy), as well as to patients with mitral stenosis.
Anaphylactoid reactions during desensitization
There are isolated reports of the development of anaphylactoid reactions in patients receiving ACE inhibitors during desensitizing therapy, for example, with hymenoptera venom. ACE inhibitors should be used with caution in patients susceptible to allergic reactions undergoing desensitization procedures. The use of ACE inhibitors should be avoided in patients receiving bee venom immunotherapy. However, this reaction can be avoided by temporarily discontinuing the ACE inhibitor before starting the desensitization procedure.
Hypersensitivity/angioedema
Angioedema of the face, extremities, lips, tongue, epiglottis and/or larynx, which may occur during any period of treatment, has rarely been reported in patients taking an ACE inhibitor, including lisinopril. In this case, treatment with the drug should be stopped as soon as possible, and the patient should be monitored until symptoms completely regress. Angioedema with laryngeal edema can be fatal. Swelling of the tongue, epiglottis or larynx can cause airway obstruction, so appropriate therapy (0.3-0.5 ml of 1:1000 epinephrine (adrenaline) solution subcutaneously) and/or measures to ensure airway patency should be immediately carried out. In cases where the swelling is localized only on the face and lips, the condition most often goes away without treatment, but the use of antihistamines is possible.
The risk of developing angioedema is increased in patients who have a history of angioedema not associated with previous treatment with ACE inhibitors.
In rare cases, intestinal angioedema develops during therapy with ACE inhibitors. In this case, patients experience abdominal pain as an isolated symptom or in combination with nausea and vomiting, in some cases without previous angioedema of the face and with normal levels of Cl-esterase. The diagnosis is made using computed tomography of the abdominal cavity, ultrasound, or at the time of surgery. Symptoms disappear after stopping ACE inhibitors. In patients with abdominal pain taking ACE inhibitors, the possibility of developing angioedema of the intestine must be taken into account when making a differential diagnosis.
Concomitant use with mTOR inhibitors
(mammalian Target of Rapamycin - target of rapamycin in mammalian cells), for example, temsirolimus, sirolimus, everolimus, racecadotril (an enkephalinase inhibitor used to treat acute diarrhea), estramustine
In patients concomitantly receiving therapy with mTOR inhibitors, racecadotril, estramustine, the risk of developing angioedema (for example, swelling of the airways or tongue with or without impairment of respiratory function) may be increased (see section "Interaction with other drugs").
Anaphylactic reactions during low-density lipoprotein (LDL) apheresis
In rare cases, life-threatening anaphylactoid reactions may occur in patients receiving ACE inhibitors during LDL apheresis using dextran sulfan. To prevent an anaphylactoid reaction, ACE inhibitor therapy should be temporarily discontinued before each apheresis procedure.
Hemodialysis
Anaphylactoid reactions have also been observed in patients on hemodialysis using high-flow dialysis membranes (eg, AN69®) who are also taking ACE inhibitors. In such cases, the use of a different type of dialysis membrane or another antihypertensive agent should be considered.
Neutropenia/agranulocytosis/thrombocytopenia/anemia
During therapy with ACE inhibitors, neutropenia/agranulocytosis, thrombocytopenia and anemia may develop. With normal renal function and the absence of other complications, neutropenia rarely occurs. ACE inhibitors are used only in emergency cases in the presence of systemic vasculitis, immunosuppressive therapy, taking allopurinol or procainamide, as well as when combining all of these factors, especially against the background of previous renal failure. There is a risk of developing severe infectious diseases that are resistant to intensive antibiotic therapy. When carrying out therapy with Lisinopril in patients with the above factors, it is necessary to regularly monitor the number of leukocytes.
Lithium preparations
The combined use of Lisinopril and lithium preparations is not recommended (see section “Interaction with other drugs”).
Dry cough
Cough has been reported when using ACE inhibitors. The cough is dry and prolonged, which disappears after stopping treatment with an ACE inhibitor. In the differential diagnosis of cough, cough caused by the use of an ACE inhibitor must also be taken into account.
Ethnic differences
It should be taken into account that patients of the Negroid race have a higher risk of developing angioedema. Like other ACE inhibitors, lisinopril is less effective in lowering blood pressure in black patients. This effect may be associated with a pronounced predominance of low-renin status in black patients with arterial hypertension.
Double blockade of the RAAS
Cases of hypotension, syncope, stroke, hyperkalemia and renal dysfunction (including acute renal failure) have been reported in susceptible patients, especially when used concomitantly with drugs that affect this system. Therefore, double blockade of the RAAS by combining an ACE inhibitor with an ARA II or aliskiren is not recommended. Combination with aliskiren and drugs containing aliskiren is contraindicated in patients with diabetes mellitus and/or moderate or severe renal impairment (GFR <60 ml/min/1.73 m2 body surface area) (see sections "Contraindications" and "Interaction" with other drugs") and is not recommended in other patients.
Concomitant use of lisinopril and ARB II in patients with diabetic nephropathy is contraindicated and is not recommended in other patients.
Potassium-sparing diuretics, potassium supplements, potassium-containing table salt substitutes and food supplements
Concomitant use with ACE inhibitors is not recommended (see section “Interaction with other drugs”).
Patients with diabetes mellitus
When using the drug in patients with diabetes mellitus receiving oral hypoglycemic agents or insulin, blood glucose concentrations should be regularly monitored during the first month of therapy.
Liver dysfunction
The use of ACE inhibitors can lead to the development of cholestatic jaundice with progression up to fulminant liver necrosis, so it is necessary to stop taking the drug if the activity of “liver” transaminases increases and symptoms of cholestasis appear.
Hyperkalemia
During therapy with ACE inhibitors, including lisinopril, hyperkalemia may develop. Risk factors for hyperkalemia include renal failure, old age, diabetes mellitus, certain concomitant conditions (for example, decreased blood volume, acute heart failure, metabolic acidosis), concomitant use of potassium-sparing diuretics (such as spironolactone, eplerenone, triamterene, amiloride), as well as potassium supplements or potassium-containing substitutes for table salt and the use of other drugs that increase the content of potassium in the blood plasma (for example, heparin). Hyperkalemia can cause serious heart rhythm problems, sometimes fatal. The simultaneous use of the above drugs should be carried out with caution.
Kidney transplant
There are no data on the use of lisinopril after kidney transplantation. Primary hyperaldosteronism
Patients with primary hyperaldosteronism are usually resistant to treatment with antihypertensive drugs that affect the RAAS. In this regard, Lisinopril is not recommended for use in such patients.
It is not recommended to drink alcohol (ethanol) during treatment with Lisinopril.
Lisinopril: a universal drug in the arsenal of a cardiologist
It became possible to effectively influence the cardiovascular continuum with the introduction of diuretics and selective beta-blockers into clinical practice. The next stage was the emergence of angiotensin-converting enzyme inhibitors (ACEIs) and calcium antagonists (CAs), which contributed to further advances in the treatment of CVD. With good reason, the last quarter of the twentieth century can be called the “era of ACE inhibitors.” Today, five main classes of antihypertensive drugs - thiazide diuretics, ACE inhibitors, angiotensin receptor blockers (ARBs) and β-blockers (BABs) - are suitable for initiation and maintenance of antihypertensive treatment in monotherapy or in combination [2 ,3]. When choosing one or another antihypertensive drug, it is necessary to remember that it must not only adequately reduce blood pressure to the target level, control it throughout the day, improving the blood pressure profile, but also meet a number of other requirements: the drug must reduce the reabsorption of Na+ and water, and not increase dysfunction endothelium, do not activate the sympathetic nervous system, have organoprotective properties, and be metabolically neutral. These requirements are fully met by ACE inhibitors, of which there are today more than 30 original drugs and their generics. Their pharmacological action is due to their influence on the functional state of the renin–angiotensin–aldosterone system (RAAS). ACE inhibitors have a highly selective action: they suppress the conversion of angiotensin I to angiotensin II without directly interacting with other components of the RAAS. Summarizing the data on the properties and mechanism of action of ACE inhibitors, we can dwell on the main protective effects of this group of drugs: 1. Cardioprotective effects (restoring the balance between the need and supply of myocardium O2, reducing pre- and afterload of the left ventricle (LV), reducing volumes and weight, slowing LV remodeling, decreased sympathetic stimulation, antiarrhythmic effect). 2. Vasoprotective effects (potentially direct antiatherogenic effect, antiproliferative and antimigration effect on smooth muscle cells, monocytes, neutrophils; improved endothelial function, antiplatelet effect, increased endogenous fibrinolysis, improved arterial compliance and tone). Among the many representatives of the ACEI class, lisinopril deserves special attention. Lisinopril (Diroton®, produced by the pharmaceutical company) is an ACE inhibitor with an extremely wide spectrum of action and original properties, which allows it to be used in a wide variety of situations. In addition, a large evidence base has been accumulated on the effectiveness and safety of lisinopril based on the results of a number of clinical studies. Lzinopril (Diroton) is the only hydrophilic ACEI, practically does not bind to plasma proteins and is not distributed in adipose tissue. The chemical structure of lisinopril contains a carboxyl group, which binds the zinc-containing domain of ACE. Unlike most ACE inhibitors, lisinopril is not a prodrug. Absorbed into the gastrointestinal tract, it does not undergo further metabolic transformations and is excreted unchanged by the kidneys. Lisinopril clearance correlates with creatinine clearance, so as creatinine clearance decreases, lisinopril excretion also decreases. In patients with renal failure, the elimination of the drug is slowed down, and therefore dose adjustment is required. The drug has fairly variable bioavailability - from 26 to 60%. Food intake does not affect the bioavailability of the drug. Its action begins 1 hour after oral administration, the peak effect develops after 4–6 hours, and the duration of action reaches 24 hours, which provides a convenient administration regimen - once a day [4]. The severity of the inhibitory effect of lisinopril on ACE activity was studied in vitro on rabbit lungs. The affinity constant of ACE for lisinopril was comparable to that of enalaprilat and captopril, but the dissociation half-life of the drugs was 105, 27 and 9 minutes, respectively. These data indicate a greater affinity of lisinopril for ACE [5]. Unlike most other ACE inhibitors, lisinopril does not contain a sulfhydryl group, which is the cause of a number of side effects (neutropenia and proteinuria) [6]. Lisinopril is used to treat patients with hypertension, congestive heart failure, and after acute myocardial infarction (AMI) [7]. Moreover, it was recently approved in a number of countries (Great Britain, Spain, Belgium) for the treatment of diabetic nephropathy, and in Mexico, Portugal and New Zealand for the treatment of patients with diabetic retinopathy [8]. Efficacy of lisinopril in the treatment of hypertension In 2002, the results of the ALLHAT (Antihypertensive and Lipid–Lowering treatment to prevent Heart Attack Trial) study were published [9], which assessed mortality from coronary artery disease and the incidence of myocardial infarction in elderly patients. In the ALLHAT study, 15,255 patients received chlorthalidone at a dose of 12.5–25 mg, 9,048 patients received amlodipine at a dose of 2.5–10 mg, and 9,054 patients received lisinopril at a dose of 10–40 mg per day. If the target blood pressure level could not be achieved, then at the next stage a second drug was added (atenolol - 25-100 mg, reserpine - 0.05-0.2 mg once a day or clonidine - 0.1-0.3 mg twice a day day). If there was no effect, hydralazine was added at the third stage - 25-100 mg twice a day. None of the three drugs was shown to be superior in their ability to prevent the primary composite endpoint of myocardial infarction and cardiovascular mortality. The analysis of overall mortality also did not reveal any benefits of any drug. Lisinopril was slightly inferior to chlorthalidone in its ability to prevent strokes, hospitalization for angina, and worsening heart failure. However, lisinopril was significantly superior to amlodipine in preventing decompensated heart failure in whites; in black patients, the effectiveness of lisinopril and amlodipine did not differ significantly. A comparison of the effectiveness of two ACEIs, enalapril and lisinopril, was carried out using blood pressure monitoring to control the effectiveness of therapy. The target BP was set to 140/90 mm Hg, and the dose of both drugs was titrated to achieve this BP level. Hydrochlorothiazide was added if necessary. Both drugs significantly reduced blood pressure, but the effect of lisinopril was more pronounced. The average doses of drugs at the end of the study were 18 mg enalapril and 8 mg hydrochlorothiazide in one group, and 17 mg lisinopril and 6 mg hydrochlorothiazide in the second group. With the same administration regimen (once daily), lisinopril has a longer duration of action. The safety of the drugs was comparable [10]. A relatively small study (65 patients with DBP 95–115 mm Hg) compared the effectiveness and tolerability of lisinopril and the b-blocker nebivolol. Lisinopril was prescribed at a dose of 20 mg once daily, nebivolol - 5 mg once daily. Both drugs caused a significant decrease in blood pressure and were well tolerated by patients [11]. A Norwegian multicenter study examined the antihypertensive efficacy, tolerability, and impact of lisinopril (mean dose 18.8 mg) and nifedipine (mean dose 37.4 mg) on quality of life in 828 patients with mild to moderate hypertension. Lisinopril was more effective in lowering blood pressure and was better tolerated by patients. Both drugs had an equally good effect on the quality of life of patients [12]. A study comparing the effectiveness of lisinopril (20 mg) and the ARB telmisartan (80 mg) included 32 previously untreated patients with hypertension. The effectiveness of the drugs was the same both according to routine office blood pressure measurements and according to 24-hour blood pressure monitoring [13]. Lisinopril demonstrated comparable efficacy to the ARB valsartan. The large randomized trial PREVAIL (Ehe Blood Pressure Reduction and Tolerability of Valsartan in Comparison with Lisinopril study) included 1213 patients with grade 1–3 hypertension (SBP 160–220 mmHg and DBP 95–110 mmHg. ). Patients were randomized to receive valsartan 160 mg or lisinopril 20 mg per day. After four weeks, if there was insufficient effectiveness, hydrochlorothiazide was added to therapy. The total duration of treatment was 16 weeks. 1100 patients completed the full course of treatment; 51 patients in the valsartan group and 62 patients in the lisinopril group discontinued treatment due to side effects of therapy. The decrease in blood pressure was identical in both treatment groups – 31.2/15.9 mmHg. and 31.4/15.9 mmHg. respectively [14]. Lisinopril for obesity Two large studies - the NHS (non-smoking women) and the Seventh–Day Adventist Study (non-smoking, non-alcoholic vegetarian men) revealed a direct correlation between body mass index and cardiovascular mortality [15]. According to the Framingham study, every 4.5 kg of weight increases systolic blood pressure by 4.4 mmHg. in men and by 4.2 mm Hg. among women. The multicenter, double-blind, randomized, placebo-controlled study TROPHY conducted a comparative study of the effectiveness of 12-week treatment of 232 obese and hypertensive patients with lisinopril and hydrochlorothiazide (HCT). ABPM data showed that lisinopril and hydrochlorothiazide effectively reduced blood pressure throughout the day compared to placebo (p < 0.001). However, a decrease in DBP below 90 mm Hg. noted in 60% of patients treated with lisinopril and only in 43% of patients treated with hydrochlorothiazide (p<0.05). It is important that the majority of patients (57%) taking lisinopril remained on a dose of 10 mg throughout the treatment period, while the majority of patients receiving HCTZ (71%) required an increase in dose to 25–50 mg per day , which is associated with extremely adverse metabolic effects. Both drugs had no significant effect on insulin levels and lipid profiles, but plasma glucose levels at 12 weeks differed significantly (p<0.001) in the lisinopril (–0.21 mmol/L) and hydrochlorothiazide (+0.31 mmol/L) groups [16 ]. It must be recalled that lisinopril is the only hydrophilic ACE inhibitor with a duration of action of 24–30 hours that is not distributed in adipose tissue. These properties allow us to consider it a drug of choice in the treatment of obese patients with hypertension. Lisinopril and nephroprotection The nephroprotective effect of lisinopril has been demonstrated at various stages of diabetic nephropathy, regardless of the presence of hypertension. The multicenter 2-year placebo-controlled trial EUCLID (Randomised placebo-controlled trial of lisinopril in normotensive patients with insulin-dependent diabetes and normoalbuminuria or microalbuminuria) examined the early administration of lisinopril on the progression of diabetic nephropathy and retinopathy in 530 patients with type 2 diabetes (T2DM). ) without hypertension with normoalbuminuria (85% of patients) and microalbuminuria (15%). The level of microalbuminuria (MAU) in the lisinopril group at the end of observation was 18.8% lower than in the placebo group. The maximum effect was found in patients who already had nephropathy at the beginning of the study: in patients with initial normoalbuminuria, the decrease in urinary albumin excretion compared to placebo was 12.7% (1.0 mcg/min.), while in patients with initial normoalbuminuria MAU – 49.7% (34.2 mcg/min.). Thus, the EUCLID study demonstrated the ability of ACE inhibitors to slow down both the development and progression of the initial stage of diabetic nephropathy. At the same time, the greatest nephroprotective properties were manifested precisely at the MAU stage. The EUCLID trial also assessed the effect of lisinopril therapy on the development and progression of diabetic retinopathy (DR) [17]. In the lisinopril group, a 50% reduction in the risk of progression of DR was detected (OR=0.5) compared with placebo, while the maximum protective effect of ACE inhibitors on the development and progression of DR (OR=0.34) was observed in patients with compensation of carbohydrate metabolism - with HbA1c level less than 7%. In one of the largest studies using lisinopril in patients with type 2 diabetes, which included 3463 patients with initial and severe diabetic nephropathy (DN) and hypertension, the administration of lisinopril even for a short period (3 months) showed not only the high antihypertensive effectiveness of the drug, but also an improvement in nitrogen excretion function of the kidneys - in almost 50% of patients with initially elevated creatinine levels, this indicator stabilized. Studies also noted a positive effect of lisinopril on indicators of metabolic control (levels of glycated hemoglobin and blood lipids) and good tolerability of therapy - side effects developed in only 2.2% of patients [18]. Lisinopril and cardioprotection The 2-year ELVERA study (Effects of amlodipine and lisinopril on Left Ventricular mass) examined the effect of lisinopril and amlodipine on myocardial mass and left ventricular diastolic function in elderly patients with hypertension who did not receive antihypertensive therapy. The study included 166 patients with hypertension (DBP 95–115 mm Hg and SBP 160–220 mm Hg) aged 60 to 75 years: 81 patients received amlodipine at a dose of 2–10 mg per day, 85 patients received lisinopril at a dose of 10–20 mg per day. Myocardial mass index (MMI) decreased by 25.7 g/m2 in the amlodipine group and by 27 g/m2 in the lisinopril group. The SAMPLE study [19] included 206 patients with hypertension and LVH. During therapy with lisinopril at a dose of 20 mg/day. in combination with HCTZ (12.5–25 mg/day) and without it, an adequate reduction in blood pressure and a decrease in LV MMI by 15.8% were observed. The effectiveness of early use of lisinopril in acute myocardial infarction (AMI) has also been proven. The results of the GISSI-3 study showed that if treatment with lisinopril begins on the first day of AMI with stable hemodynamics, there is a significant reduction in overall mortality. Nitrates did not improve these indicators. Mortality rates and combined endpoints by month 6 were significantly lower (p=0.03) in the group of patients treated with lisinopril [20]. The SMILE-2 (Survival of Myocardial Infarction) study directly compared two ACE inhibitors for AMI: zofenopril at a dose of 30–60 mg and lisinopril at a dose of 5–10 mg per day. Both drugs were prescribed to patients who received thrombolytic therapy for AMI. ACE inhibitor therapy began no later than 12 hours after completion of thrombolysis and lasted 42 days. A total of 1024 patients were included in the study. There were no significant differences in the risk of cardiovascular complications in both treatment groups [20]. Lisinopril for CHF The randomized ATLAS study compared the effectiveness and tolerability of long-term therapy with low (2.5–5 mg) and high (32.5–35 mg) doses of lisinopril in 3164 patients with class II–IV CHF and an ejection fraction of no more than 30% . During observation, in the group of patients receiving high doses of lisinopril, there was a decrease in mortality from all causes by 8% and mortality from cardiovascular causes by 10%. In addition, therapy with high doses of lisinopril led to a significant reduction in the need for hospitalization due to decompensated CHF (by 24%) [22]. In addition, the ATLAS study established an extremely favorable economic effect when using high doses of the drug - the cost of treatment was $2 billion/year lower [23]. One of the most worthy representatives of lisinopril on the Russian market, completely satisfying the price-quality ratio, is lisinopril - Diroton. In Volgograd, under the guidance of prof. S.V. Nedogoda [24] conducted a 6-month open randomized study comparing the clinical and pharmacoeconomic effectiveness of lisinopril - Diroton (5 mg N 28) and Lizoril (10 mg N 30) in 40 patients with hypertension. It was proven that Diroton reduced both SBP and DBP to a slightly greater extent than Lizoril (p>0.05), but at the same time, the cost-effectiveness ratio of Diroton was 1.6 times better than that of Lizoril. Thus, lisinopril (Diroton) is an extremely effective and economical antihypertensive drug with organoprotective properties, which is convenient for doctors to work with in a wide variety of clinical situations. References 1. MRFIT research group. Multiple Risk Factor intervention Trial. Risk factor changes and mortality results. JAMA 1982;248:1465–77 2. The Task Force for the management of arterial hypertension of the European Society of Hypertension and of the European Society of Cardiology. 2007 Guidelines for the management of arterial hypertension. J Hypertens 2007; 25:1105–1187. 3. VNOK. Prevention, diagnosis and treatment of arterial hypertension. Russian recommendations (second revision). Cardiovascular Therapy and Prevention 2004. Appendix 4. 4. Semple PF et al. Onset of action of captopril, enalapril, enalaprilic acid and lisinopril in normal man // Cardiovascular Drugs and Therapy. – 1987– Vol. 1. – P. 45–50. 5. Bull HG Inhibition of rabbit lung ACE by lisinopril, enalapril and captopril. // J. of Biological Chem. –1985–Vol.260 – P.2952–2962. 6. Chodoff L. Lisinopril: a new ACE inhibitor for the treatment of hypertension and congestive heart failure // Mt. Sinai. J. Med. – 1990. – Vol. 57. – P. 169–171. 7. Lisinopril. Mosby's GenRx – The complete reference for generic and brand drugs. 9th edn., Mosby, Inc., St. Louis, Missouri, 1999. 8. AstraZeneca. 'Zestril' prescribing information. . Available from: https://www.zestrilinfo.com/info/info.htm. 9. Davis BR, Culter JA, Gordon DJ Antihypertensive and lipid–lowering treatment to prevent heart attack trial. Am. J. Hypertens. – 1996. –Vol.9. –P.342–60. 10. Diamant M, Vincent HH Lisinopril versus enalapril: evaluation of through: peak ratio by ambulatory blood pressure monitoring. J Hum Hypertens. – 1999/ – Jun;13(6):405–12. 11. Rosei EA, Rizzoni D, Comini S et al. Evaluation of the efficacy and tolerability of nebivolol versus lisinopril in the treatment of essential arterial hypertension: a randomized, multicentre, double-blind study. Blood Press Suppl. – 2003, May;1:30–5 12. Os I, Bratland B, Dahlof B at al. Lisinopril or nifedipine in essential hypertension? A Norwegian multicenter study on efficacy, tolerability and quality of life in 828 patients. J Hypertens. – 1992, Feb;10(2). 13. Stergiou GS, Efstathiou SP, Roussias LG et. Al. Blood pressure– and pulse pressure–lowering effects, through:peak ratio and smoothness index of telmisartan compared with lisinopril. J Cardiovasc Pharmacol. – 2003, Oct;42(4):491–6. 14. Malacco E, Santonastaso M, Vari NA et al. Comparison of valsartan 160 mg with lisinopril 20 mg, given as monotherapy or in combination with a diuretic, for the treatment of hypertension: the Blood Pressure Reduction and Tolerability of Valsartan in Comparison with Lisinopril (PREVAIL) study. Clin Ther. – 2004. – Jun;26(6):855–65. 15. Lindsted K. Study Seventh–Day Adventist. Int J Obesity, 1991. 16. Reisin E et al. Lisinopril versus HCTZ in obese hypertensive patients: a multicenter placebo–controlled trial. Treatment in obese Patients with Hypertension (TROPHY) Study Group. Hypert 1997: Jul 30: 140–145 17. Euclid Study Group. Effect of Lisinopril On ProgReSSion of Retinopathy in Normotensive Peopler with Type 1 Diabetes. Lancet 1998; 351: 28–31. 18. Parting hh. Effects of Ace Inhibitors On Renal Function InCipient and Overtic Nephropathy. // J Diabetes Complications. 1996; 10 (3): 133–135. 19. Mancia G., Zanchetti A. et al. Study on Monitoring of Blood Pressure and Lisinopril EvalUation. Circulation, 1997; 95 (6); 1464–70. 20. Latini R., Nicolosi G., Maggioni Ap. et al. The Beneficial Effect of Lisinopril on Left Ventricular Remodeling After a First Myocardial IS Modulated by Age. The Gissi -3 Echo Database (abstract) no. 775–11. .1 Am Coll Cardiol 1996; 27 (2) SUPPL. A: 281 A 21. Ambrossioni E., Borgii S., Magnani V. et al. The Effect of the Angiotensin - Convertmg - Enzmie Inhibitor Zofenopril On Mortaly and Morbidity Anterior MyCardial Infarction. New Engi J Med. 1995; 332: 2: 80–85. 22. Packer M., Poole - Wilson P., Armstrong P. et al. Comparative Effects of Low - Dose Versus High - Dose Lisinopril on Survival and Major Events in Chronic Heart Failure: The Assessement OF TREATMENTH LISINOPRI ). Europ. Heart J., 1998; 19 (SUPPL.): 142 (abstract). 23. Packer m et al. Comparative Effects of Low and High Doses of the Acei Lisinopril, On Morbidity and Mortaly in Chronic Heart Failure. Circulation 1999; 100: 1–7. 24. Ostroumova O.D., Nedogoda S.V., Mamaev V.I., Shorikova E.G., pharmacoeconomic aspects of the effectiveness of angiotensic enzyme inhibitors in arterial hypertension and heart failure, rmzh volume 11, No. 5, p. 262– 267
Lisinopril in the treatment of arterial hypertension in patients with pathology of the digestive system
The prevalence of arterial hypertension (AH) in Russia reaches 40% in men and 50% in women. In 83.3% of patients, hypertension is combined with diseases of the digestive system, including 30% with liver pathology.
To correct blood pressure (BP) in patients with hypertension, antihypertensive drugs of various pharmacological groups are used, among which the most frequently prescribed drugs are angiotensin-converting enzyme (ACE) inhibitors [7].
Over the past 30 years, ACE inhibitors have been called the “cornerstone of the treatment of cardiovascular diseases” (E. Braunwald, 1991) and the “gold standard of therapy” (J. Cohn, 1998) [10].
The results of numerous international studies have shown that these drugs are the most effective, reducing mortality from cardiovascular diseases, having organoprotective effects, and therefore are recommended as first-line antihypertensive drugs for long-term treatment of patients with hypertension.
Currently, the best known ten ACE inhibitors are captopril, enalapril, benazepril, fosinopril, lisinopril, moexipril, perindopril, quinapril, ramipril and trandolapril. Five of them (captopril, enalapril, lisinopril, ramipril, trandolapril) have been shown to reduce mortality in large studies [21, 23, 24, 28–32].
The Scandinavian study (STOP-2) compared the effectiveness of ACE inhibitors (lisinopril or enalapril 10 mg per day) with other antihypertensive drugs (beta-adrenergic blockers, hydrochlorothiazide in combination with amiloride or felodipine) in the treatment of 6614 patients with hypertension for 54 months [ 22].
This study showed that ACE inhibitors significantly reduced the risk of heart failure.
The ALLHAT study included 33,357 hypertensive patients followed for an average of 4.9 years [1, 17]. The first group of patients was prescribed chlorthalidone (up to 25 mg per day), the second - amlodipine (up to 10 mg per day), the third - lisinopril (up to 40 mg per day). During therapy with lisinopril, stroke (“end point”) occurred less frequently than when using a diuretic.
The TPOPHY study compared the effectiveness of monotherapy with hydrochlorothiazide and lisinopril in overweight hypertensive patients. Monotherapy with an ACE inhibitor ensured blood pressure control in 60%, and monotherapy with a diuretic in 43% of patients. In the group receiving lisinopril, in more than half, a dose of 10 mg/day was sufficient, and only one in four needed to be prescribed 40 mg/day. To achieve the target blood pressure level during diuretic therapy, almost every second person required the prescription of 50 mg/day of hydrochlorothiazide, which is associated with the likelihood of life-threatening arrhythmias.
In the GISSI-3 study, patients receiving lisinopril had a significantly reduced risk of death and cardiovascular disease [9].
Therapy with ACE inhibitors in patients with hypertension and diabetes mellitus significantly reduces the risk of target organ damage. In the EUCLID study in 530 patients with type 1 diabetes mellitus, lisinopril had a nephroprotective effect and reduced the risk of progression of retinopathy.
The multicenter, randomized, double-blind ATLAS trial showed that treatment with high doses (33.2 mg per day) of lisinopril was associated with a significant reduction in the risk of death or hospitalization by 12% [27].
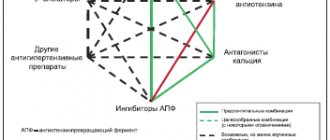
The antihypertensive effect of ACE inhibitors is associated with:
- inhibition of the renin-aldosterone-angiotensin system in tissues and the vascular wall;
- inhibition of the conversion of inactive angiotensin I into the active vasoconstrictor angiotensin II and a decrease in aldosterone secretion;
- increased plasma renin activity;
- accumulation of bradykinin due to inhibition of kininase II;
- dilatation of renal vessels with increased natriuresis;
- increased synthesis of prostaglandins PGI2 and PGE2 [18, 20].
The release of PGI2 and PGE2 has vasodilatory, diuretic and natriuretic effects. Treatment with ACE inhibitors also reduces the formation of other vasoconstrictor and antinatriuretic substances, such as norepinephrine, arginine vasopressin, and endothelin-1 [12].
Hemodynamic effects when using ACE inhibitors are manifested:
- a decrease in total vascular resistance due to an indirect vasodilating effect, which leads to a decrease in blood pressure by 15–25%;
- relaxation of volume vessels with a decrease in filling pressure of the left ventricle;
- increased minute blood volume;
- an increase in renal blood flow due to dilatation of efferent arterioles in the glomeruli [11].
Classification of ACE inhibitors. Despite the common mechanism of action, ACE inhibitors differ in chemical structure, the presence of additional functional groups in the molecule, the nature of the prodrug, activity and pharmacokinetic profile, which is very important to consider when treating patients with various pathologies of the digestive organs [3].
The most popular chemical classification, according to which drugs are divided into four main classes depending on which chemical group in their molecule binds to the zinc ion in the active centers of the angiotensin I-converting enzyme:
- preparations containing a sulfhydryl group;
- drugs containing a carboxyl group;
- preparations containing a phosphinyl group;
- drugs containing the hydroxamic group [15].
Analysis of literature data shows that according to the duration of the antihypertensive effect, ACE inhibitors can be divided into two groups:
- medium duration - captopril;
- long-acting - enalapril, lisinopril, quinapril, which in most cases provide round-the-clock control over blood pressure levels when taken once a day.
Taking into account data on physicochemical properties and pharmacokinetic characteristics, ACE inhibitors are divided into three classes:
1. Lipophilic ACE inhibitors (captopril), which themselves have pharmacological activity, but undergo further transformations in the liver to form pharmacologically active disulfides, which are eliminated by renal excretion.
2. Lipophilic prodrugs (pharmacologically inactive) become active diacid metabolites after metabolic transformation in the liver (enalapril to enalaprilat), which are then transformed into inactive compounds [13]. In patients with liver pathology, both of these processes are impaired, and with a decrease in blood flow in the liver, there is a delay in the conversion of the prodrug to its active form during the first passage through it.
Accordingly, in liver diseases, drugs that require transformation to acquire activity have a weaker effect [6, 14].
Prodrugs are more lipophilic than their pharmacologically active metabolites, which allows them to ensure rapid and complete absorption when taken orally.
ACE inhibitors of this class should be divided into three subgroups depending on the predominant route of elimination of their active diacid metabolites:
- subclass A - drugs with predominantly renal elimination;
- subclass B - drugs with two main elimination routes;
- subclass C - drugs with predominantly hepatic elimination.
3. Hydrophilic drugs (lisinopril), which are not metabolized in the patient’s body. They circulate in the blood in a form unbound to plasma proteins and are eliminated unchanged through the kidneys. Their concentration in the blood plasma is determined by the dose taken orally, as well as the rate of absorption and the rate of excretion through the kidneys [16, 19, 26].
Only four ACE inhibitors (captopril, libenzapril, lisinopril and ceronapril) directly have biological activity. All other ACE inhibitors themselves are inactive substances or pro-drugs, i.e., they exhibit their effect after biotransformation in the liver and the formation of active metabolites [25].
The degree of blocking of the tissue renin-angiotensin system by various ACE inhibitors varies. Drugs that are characterized by less lipophilicity cause less accumulation of bradykinin in tissues, which is associated with the appearance of a side effect - dry cough.
Lisinopril is a well-studied ACE inhibitor, the benefit of which has been proven in the treatment of patients with hypertension.
Lisinopril is an active pharmacological compound.
Lisinopril was the third ACE inhibitor (after captopril and enalapril) of the drugs in this group that entered clinical practice.
Lisinopril has a prolonged antihypertensive effect. The onset of the antihypertensive effect is 1–3 hours after oral administration, the peak of action is after 6 hours, the duration of action is 24 hours with a stable effect after 2–4 weeks of treatment [4, 5].
The antihypertensive effect lasts more than a day. In case of abrupt cessation of therapy with lisinopril, there is no sudden increase in blood pressure, as well as a significant increase in blood pressure values before the start of treatment.
Lisinopril causes dilatation of arterioles and veins, which leads to a decrease in blood pressure by approximately 15% due to a decrease in total peripheral vascular resistance. Lisinopril does not cause reflex tachycardia due to stimulation of the vagus nerve and a decrease in the sensitivity of carotid sinus baroreceptors due to improved compliance and dilatation of the carotid artery.
Pharmacokinetics
After oral administration, the bioavailability of lisinopril is 25–29%. The functional state of the liver does not affect bioavailability. Eating does not change the absorption of the drug from the gastrointestinal tract. In the human body it is not metabolized and is excreted unchanged in the urine. In blood plasma, lisinopril does not bind to proteins. The half-life is 12.6 hours. The drug undergoes glomerular filtration, is secreted and reabsorbed in the tubules. The maximum concentration is achieved 6 hours after taking a single dose, and the steady-state level of concentration with regular use is achieved after 2-3 days.
For hypertension, the initial dose is 10 mg/day for a single dose, followed by a possible gradual increase to 40 mg/day.
Thus, when treating patients with hypertension with pathology of the digestive organs, the doctor has the opportunity to choose a drug from various classes of ACE inhibitors, depending on their pharmacokinetic characteristics.
In our work, we assessed the effectiveness of an ACE inhibitor (lisinopril) in the treatment of patients with hypertension with various pathologies of the digestive organs.
Purpose of the study
To evaluate the pharmacodynamics of lisinopril in various diseases of the digestive system in patients with hypertension.
Materials and research methods
The study included 60 patients with hypertension in combination with steatosis (group 1), liver cirrhosis (group 2), duodenal ulcer (group 3), 20 people in each group, respectively.
Titration of lisinopril dosages was carried out weekly under the control of 24-hour blood pressure monitoring (ABPM). Based on complaints, medical history and examination (blood tests, esophagogastroduodenoscopy, ultrasound examination of the abdominal organs), the presence of pathology in the liver and upper digestive tract was established. Patients with duodenal ulcer with normal liver function constituted the comparison group (Table 1).
To assess the effectiveness of lisinopril, ABPM was carried out using the ABRM-02 monitor using the oscillometric method of measuring blood pressure in a free motor mode. Registration was carried out on the “non-working” arm in the absence of blood pressure asymmetry. If blood pressure asymmetry is more than 5 mm Hg. Art. the study was conducted on the arm with higher scores. Blood pressure measurements were carried out for 24 hours every 15 minutes from 6.00 to 22.00 hours and every 30 minutes from 22.00 to 6.00 hours.
In order to clarify the daily blood pressure profile and assess the hypotensive effect of lisinopril, average blood pressure values were determined based on ABPM data. Normally, during the daytime, blood pressure should not exceed 140 and 90 mmHg. Art., at night - 120 and 80 mm Hg. Art. As an indicator of pressure load, we assessed the time index (TI) - the percentage of time during which blood pressure exceeds the critical level for certain time periods (in accordance with the recommendations of the American Society of Hypertension, a VI of more than 30% indicates the presence of elevated blood pressure) [3].
Statistica 5.0 program was used for statistical data processing. For each indicator, the mean value and standard deviation from the mean value were calculated. The statistical significance of changes in indicators was determined using Fisher's test. Differences were considered statistically significant at p < 0.05.
Results and its discussion
According to ABPM data, a persistent increase in blood pressure was initially detected in all examined patients; no significant differences were found between the groups.
The effectiveness of therapy was assessed after 1, 2, 3 and 4 weeks according to the VI ABPM level: good - with VI less than 30%, unsatisfactory - with VI more than 30%.
If lisinopril was insufficiently effective, the dose was gradually increased to 20 mg (Tables 2, 3).
As can be seen from table. 2 and 3, during therapy with lisinopril at a dose of 10 mg/day, a decrease in average daily blood pressure and blood pressure values was noted in all three groups. When treated with lisinopril, a good antihypertensive effect was obtained in 50% of patients with liver cirrhosis. In 60% of patients with duodenal ulcer, a good effect was achieved when taking 10 mg/day, in 30% of patients - 20 mg/day.
Insufficient effectiveness of lisinopril therapy was observed in 15% of cases in patients with liver cirrhosis and in 10% in patients with duodenal ulcer, even when the dose of the drug was doubled.
Conclusion
The results of this study indicate that the effectiveness of lisinopril monotherapy did not depend on the severity of liver changes in patients with hypertension.
In this regard, the use of antihypertensive drugs that are not metabolized in the liver, which can provide adequate blood pressure control for 24 hours in hypertensive patients with gastrointestinal pathology, becomes especially relevant [2, 8].
Thus, lisinopril is a highly effective antihypertensive drug in the treatment of patients with various pathologies of the digestive system.
Literature
- Belenkov Yu. N., Mareev V. Yu., Ageev F. T. Angiotensin-converting enzyme inhibitors in the treatment of cardiovascular diseases (quinapril and endothelial dysfunction). M., 2001. 86 p.
- Drapkina O. M., Mayevskaya M. V., Korneeva O. N., Tutnov D. A., Ivashkin V. T. Clinical study of the effectiveness and safety of dapril (lisinopril) in liver pathology and concomitant arterial hypertension // Russian Medical News . 2004, No. 2, p. 39–42.
- Kobalava Zh. D. Kotovskaya Yu. V., Khirmanov V. N. Blood pressure in research practice. Ed. V. S. Moiseeva, R. S. Karpova. M.: Reafarm, 2004. 384 p.
- Komissarenko I. A., Lazebnik L. B., Mikheeva O. M. Features of the metabolism of antihypertensive drugs in patients with pathology of the digestive organs // Cardiovascular therapy and prevention. Appendix 1. 2009. 8 (6). P. 239.
- Komissarenko I. A., Mikheeva O. M., Drozdov V. N., Petrakov A. V., Silvestrova S. Yu. The use of angiotensin-converting enzyme inhibitors in patients with arterial hypertension against the background of liver pathology // Consilium medicum. 2007. T. 9. No. 11. P. 72–75.
- Kushakovsky M. S. Hypertension. St. Petersburg: Sotis, 1995. pp. 243–253.
- Lazebnik L. B., Drozdov V. N. Diseases of the digestive organs in the elderly. Anacharsis, 2003, pp. 37–39.
- Lazebnik L. B., Mikheeva O. M., Komissarenko I. A., Drozdov V. N., Petrakov A. V., Silvestrova S. Yu. Features of treatment of patients with hypertension with ACE inhibitors for pathology of the digestive organs // Experimental and Clinical gastroenterology, 2007. No. 4. pp. 47–55.
- Mazur N. A. Efficacy of non-lipophilic angiotensin-converting enzyme inhibitors in the treatment of cardiovascular diseases // Russian Journal of Cardiology. 2003. No. 4 (42). pp. 76–79.
- Mareev V. Yu. Application of ACE inhibitors in the treatment of cardiovascular diseases in the 21st century. Why is it beneficial to choose fosinopril? Heart diseases. Guide for doctors. Ed. R. G. Oganova, I. G. Fomina. M.: Litterra, 2006. pp. 3–8.
- Metelitsa V.I. Handbook of clinical pharmacology of cardiovascular drugs. St. Petersburg - M.: Binom. 2nd ed. 2002. 925 p.
- Preobrazhensky D.V., Sidorenko B.A. Treatment of arterial hypertension. M., 1999. pp. 126–136.
- Savenkov M.P., Ivanov S.N., Botsoeva M.A., Mikhailusova M.P. Correction of high blood pressure in the morning with the help of ACE inhibitors // Gedeon Richter in the CIS. 2001. No. 4 (8). pp. 27–30.
- Savenkov M.P., Ivanov S.N., Solomonova L.A., Savenkova A.M. Morning begins with dawn and increased blood pressure // Russian Medical Journal. 2006. T. 14, No. 10. P. 734–736.
- Storozhakov G.I. ACE inhibitors: place in the treatment and prevention of cardiovascular diseases // Consilium medicum. Extra edition. 2002, January, p. 3–4.
- Tkhostova E. B. Clinical effectiveness of lisinopril in patients with cardiovascular diseases // Gedeon Richter in the CIS. 2001. No. 4 (8). pp. 23–25.
- ALLHAT Authors. Major Outcomes in High-Risk Hypertensive Patients Randomized to Angiotensin-Converting Enzyme inhibitor or Calcium channel blocker VS Diuretic. JAMA, December 18, 2002, 288, 23, 2981–2996.
- Campbell DJ, Kladis A., Duncan AM Effects of converting enzyme inhibitors on angiotensin and bradykinin peptides // Hypertens. 1994, 23: 439–449.
- Choodoff L. Lisinopril: a new ACE inhibitor for the treatment of hypertension and congestive heart failure // Mt. Sinai. J. Med. 1990. Vol. 57. P. 169–171.
- Furberg CD, Pitt B. Are all angiotensin-converting enzyme inhibitors interchangeable? // Am Coll Cardiol. 2001, 37, 1456–1460.
- Gruppo Italiano per to Studio della Sopravvivenza nell'Infarto Miocardico (GISSI-3). Effects of lisinopril and transdermal glyceryl trinitrate singly and together on six-week mortality and ventricular function after acute myocardial infarction // Lancet. 1994; 343:1115–1122.
- Hansson L., Lindholm LH, Ekbom T. et al. Randomized trial of old and new antihypertensive drugs in elderly patients: cardiovascular mortality and morbidity the Swedish Trial in Old Patients with Hypertension-2 Study // Lancet. 1999, 354, 1751–1756.
- ISIS-4 (Fourth International Study of Infarct Survival) Collaborative Group (ISIS-4). A randomized factorial trial assessing early oral captopril, oral mononitrate and intravenous magnesium sulphate in 58,050 patients with suspected acute myocardial infarction // Lancet. 1995; 345:669–685.
- Kober L. et al. for the Trandolapril Cardiac Evaluation (TRACE) Study Group // N Engl J Med. 1995: 333: 1670–1676.
- Lancaster SG, Todd PA Lisinopril: a preliminary review of its pharmacodynamic and pharmacokinetic properties and therapeutic use in hypertension and congestive heart failure // Drugs. 1988, 35: 646–669.
- Opie HL Angiotensin-converting enzyme inhibitors. Wiley-Liss-Authors Publishing. New York, 1992.
- Packer M., Poole-Wilson PA, Armstrong PW et al. Comparative effects of low and high doses of angiotensin-converting enzyme inhibitor, lisinopril, on morbidity and mortality in chronic heart failure. ATLAS Study Group // Circulation. 1999; 100(23):2312–2318.
- Pfeffer MA et al. On behalf of the SAVE Investigators. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction: results of the Survival And Ventricular Enlargement trial // N Engl J Med. 1992; 327:669–677.
- The Acute Infarction Ramipril Efficacy (AIRE) Study Investigators. Effect of ramipril on mortality and morbidity of survivors of acute myocardial infarction with clinical evidence of heart failure // Lancet. 1993; 342:821–828.
- The CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS) // N Engl J Med/1987; 316:1429–1435.
- The Heart Outcomes Prevention Evaluation Study Investigators. Effects of an angiotensin-converting enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients // N Engl J Med. 2000; 342:145–153.
- The SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart future // N Engl J Med. 1991; 325; 293–302.
L. B. Lazebnik, Doctor of Medical Sciences, Professor O. M. Mikheeva, Doctor of Medical Sciences, Professor I. A. Komissarenko, Doctor of Medical Sciences, Professor
State Budgetary Educational Institution of Higher Professional Education MGMSU Ministry of Health and Social Development of Russia, Central Research Institute of Gastroenterology of the City Health Department, Moscow
Contact information for authors for correspondence