The acid-base balance in the blood is a vital parameter. Its normal values range from 7.35 to 7.45 on the pH scale. If the pH drops below 7.35, the patient has acidosis. If it rises above 7.45, this means that the patient has alkalosis.
Alkalosis and acidosis are metabolic (metabolic) and respiratory (respiratory). It depends on the reasons for their development.
Respiratory acidosis develops when a lot of carbon dioxide accumulates in the blood, forming carbon dioxide when combined with water. Because of this, the acidity of the blood increases. This condition can develop with respiratory disorders that cause decreased pulmonary ventilation.
Pathogenesis
The main pathogenetic feature of alkalosis is an excessive increase in the level of HCO3 in relation to PCO3 in the blood serum, as well as in the entire extracellular space. When metabolic acidosis is able to be compensated by hyperventilation capabilities, then not only HCO3 in the bloodstream decreases, but also a corresponding decrease in PCO3 occurs, however, in cases of severe metabolic alkalosis, such compensation (increased HCO3 - increased PCO3) is often impossible. This ratio is disrupted and the acid balance of the blood increases above normal values. Due to the increased affinity of oxygen for hemolobin and the presence of CO2 in tissues, there is a lack of oxygen supply to all tissues and organs, which explains the high mortality rate.
Over-increased activity of the respiratory center and the development of hyperventilation can develop under the influence of intense reflex excitations from baroreceptors and chemoreceptors in the vascular bed and in the higher parts of the brain. An example is acute blood loss , irritation of the sinocarotid glomeruli caused by hypoxia , excitement, exposure to N-cholinomimetics (including cititon , lobeline ), severe pain not associated with respiratory movements, as well as neurotic conditions, for example, hysterical neurosis .
Respiratory alkalosis, especially developing as a result of hypocapnia , is accompanied by general and regional hemodynamic disorders. At the same time, cerebral and coronary circulation worsens, blood pressure and minute blood volume decrease, neuromuscular excitability, muscle hypertonicity increase, and even convulsions and tetany ; intestinal motility is often inhibited and constipation develops. Of particular danger is a decrease in the activity of the respiratory center. In addition, gas alkalosis is characterized by a decrease in mental abilities, accompanied by attacks of dizziness and fainting.
3. Symptoms and diagnosis
The most common mechanism for the development of the clinical picture is circulatory disorders: blood pressure and blood supply to the heart and brain decrease. Muscle spasms and cramps, fainting, disturbances in intestinal motility, and a significant decrease in mental productivity may occur.
With gas alkalosis, changes in mental status can also be observed: agitation, excitement, etc.; Individuals with lesions of the central nervous system and a predisposition to epilepsy often develop epileptic seizures.
Non-gas types of alkalosis are characterized by severe weakness, increasing fatigue, headache, thirst with lack of appetite, sometimes convulsions or tremors; in chronic forms, skin itching, apathy, and pathological changes in the kidneys may develop.
The diagnosis is suggested clinically and confirmed laboratory.
About our clinic Chistye Prudy metro station Medintercom page!
Classification
The state of alkalosis can be compensated or decompensated, when the internal forces of the body are not able to cope with the accumulated acid-base imbalance:
- the compensated state of alkalosis is characterized by maintaining the alkaline-acid balance and blood pH within acceptable normal values - from 7.35 to 7.45, which is accompanied by shifts in buffer systems and the preservation of physiological regulatory mechanisms;
- with decompensated alkalosis, the pH rises above 7.45, which is caused by a significant increase in base concentrations and the inability of the body systems to keep physicochemical and physiological regulatory mechanisms normal.
Depending on the origin, there are respiratory (respiratory) and metabolic alkalosis .
Respiratory alkalosis
The pathology develops as a result of hyperventilation of the lungs caused by excessive release of carbon dioxide. In this case, the partial pressure of carbon dioxide in the arterial blood flow drops below 35 mmHg. Art., in this case they talk about the phenomenon of hypocapnia . The causes are usually organic brain damage due to encephalitis and neoplasms, as well as effects on the respiratory center of various toxic and pharmacological substances, including toxins, microbes, the effects of caffeine and corazol , the effects of elevated temperatures and acute blood loss. Respiratory alkalosis can even be caused by gram-negative sepsis or liver failure .
Respiratory alkalosis
If, after an acute decrease in CO2, the secretion of H+ in the distal parts of the nephron decreases over several hours and the concentrations of plasma bicarbonates decrease, then it is believed that acute respiratory alkalosis has become chronic.
Metabolic alkalosis
Metabolic alkalosis itself develops as a result of electrolyte disturbances, including hemolysis and postoperative severe conditions caused by extensive surgical interventions.
With peptic ulcers and long-term intake of large quantities of alkaline and dairy products, milk-alkali Burnett syndrome , which is characterized by general weakness, causeless nausea and vomiting, decreased appetite, aversion to dairy foods, lethargy, apathy, itching, even ataxia and deposits calcium salts in the conjunctiva, cornea and renal tubules, which initiates the gradual development of renal failure .
Forms of metabolic alkalosis
Depending on the clinical picture, 3 forms of metabolic alkalosis can be distinguished:
- mild form - in which there is a short-term increase in the content of bicarbonates in the blood, it does not require special treatment;
- moderate - characterized by an increase in the level of HCO3 to 30-40 millimol/l and is characterized by a fairly mild clinical course, the condition can be called chloride-dependent alkalosis, which is characterized by a decrease in blood chlorides to 90 millimol/l or more, and can also be caused by loss of chlorides;
- severe clinical manifestations are usually accompanied by an increase in the HCO3 content in the bloodstream by more than 50 millimol/l and an increase in blood pH - up to approximately 7.6, this condition is particularly difficult to explain both the pathogenesis and the possible causative uncompensated processes.
Chloride-independent alkalosis is characterized by an increase in extracutaneous volume and loss of K and Mg ions, which are most often observed after discontinuation of corticosteroid .
Alkalosis is a violation of the acid-base balance in the body, characterized by an excess of bases (substances that have alkali properties) in the blood.
Bases (alkalis) are substances that are capable of attaching hydrogen ions. An indicator reflecting the acidity or alkalinity of solutions is pH. The higher the concentration of bases in the blood, the higher the pH level.
Normal blood pH ranges from 7.36 to 7.44. An increase in this indicator above 7.44 indicates alkalosis.
The occurrence of various biochemical processes and the operation of enzyme systems require the presence of certain constant conditions in the body. One of them is acid-base balance.
This parameter is maintained due to the work of the lungs, kidneys, and the activity of buffer systems in the blood (chemicals that help equalize the acid-base balance).
Depending on the causes of occurrence, alkalosis can be respiratory (respiratory) and metabolic (metabolic).
The leading mechanism for the development of respiratory alkalosis is an increase in pulmonary ventilation. Due to this, the concentration of carbon dioxide and carbon dioxide in the blood decreases, which causes a shift in blood pH towards the alkaline side.
The occurrence of metabolic alkalosis can be caused by large losses of acidic gastric contents (for example, with profuse and frequent vomiting), the use of certain diuretics, disruption of the adrenal glands (organs that synthesize hormones that affect water-salt and other types of metabolism), excessive intake of the body of substances with alkaline properties (for example, when correcting acidosis).
Treatment is primarily aimed at treating the underlying disease that caused the development of alkalosis. To correct the blood pH level, intravenous infusions of acidic solutions may be required.
In respiratory alkalosis, breathing with gas mixtures containing carbon dioxide is used, which helps normalize the acid-base balance.
Synonyms Russian
Metabolic alkalosis, respiratory alkalosis.
English synonyms
Alkalosis, Metabolic Alkalosis, Respiratory Alkalosis.
Symptoms
Symptoms are often masked by manifestations of the underlying disease, which caused the development of alkalosis.
Alkalosis may have the following symptoms:
- headache
- general weakness, lethargy
- dizziness
- disturbance of consciousness up to coma
- rave
- cramps in various muscle groups
- tetany (prolonged, strong convulsive muscle contractions)
- chest pain
- heart rhythm disorder
General information about the disease
Alkalosis is a violation of the acid-base balance in the body, characterized by an excess of alkalis in the blood.
Depending on the reasons that caused its development, alkalosis can be respiratory (respiratory) and metabolic (exchange).
Metabolic processes in the body occur with the consumption of oxygen and the formation of carbon dioxide. During breathing, excess carbon dioxide is removed through the lungs and the blood is enriched with oxygen.
For normal life, it is necessary to maintain certain concentrations of oxygen and carbon dioxide in the blood. Carbon dioxide, when combined with water, forms carbon dioxide, so its increased losses with increased and deepening of breathing can lead to the development of alkalosis.
With respiratory alkalosis, a decrease in the concentration of carbon dioxide leads to a narrowing of the blood vessels in the brain, which negatively affects its blood supply. This is associated with the appearance of symptoms such as dizziness, fainting, and impaired consciousness.
These and other conditions can lead to an increase in pulmonary ventilation and the development of respiratory alkalosis:
- severe pain from the nervous system
- excitement, anxiety
- psychosis
- increase in body temperature
- cerebrovascular accident (stroke)
- brain injury
- brain tumors
- from the lungs pneumonia
- bronchial asthma
- Chronical bronchitis
- pneumothorax (a collection of air in the chest that compresses the lung)
- other causes of sepsis – penetration of pathogenic microorganisms into the blood and spread of infection throughout the body
- heart failure
- liver failure
One of the causes of metabolic alkalosis is the loss of large amounts of gastric contents (for example, with frequent vomiting). Gastric juice is acidic due to the hydrochloric acid it contains, which is necessary for digesting food. As a result, the release of stomach contents can lead to metabolic alkalosis.
The use of certain diuretic drugs can cause the development of acalosis as a result of disturbances in the water and electrolyte balance in the body.
Metabolic alkalosis can occur during various pathological processes that lead to changes in the concentration of electrolytes in the body. For example, excessive formation of the hormone aldosterone causes the excretion of potassium and hydrogen ions in the urine, and the retention of sodium and water in the body. The release of hydrogen ions leads to an increase in blood pH (alkalosis).
Aldosterone is secreted by the adrenal glands (paired organs that secrete hormones necessary to regulate metabolism and water-electrolyte balance in the body). Increased secretion of aldosterone can be associated both with diseases of the adrenal glands (primary hyperaldosteronism) and with other pathological processes in which there is a decrease in the volume of fluid in the bloodstream (for example, with heart failure, cirrhosis of the liver).
At the same time, the resulting changes in water-electrolyte balance and blood pH lead to serious disruptions in the functioning of various organs and systems. For example, a decrease in potassium levels causes general weakness, muscle pain, and heart rhythm disturbances. Due to a decrease in calcium concentration, painful muscle cramps may occur before the development of attacks of tetany (strong and prolonged convulsive contractions, spasms of various muscles).
Treatment of alkalosis is conservative and consists of compensating the patient’s condition for the underlying disease, correcting the blood pH level and water and electrolyte disturbances by intravenous administration of special solutions.
In case of respiratory alkalosis, therapeutic measures are primarily aimed at normalizing the rhythm and depth of breathing.
Who is at risk?
Risk groups include:
- persons taking certain medications (eg, diuretics, aspirin)
- persons with damage to the nervous system (traumas, strokes, brain tumors)
- people who have lost large amounts of gastric contents (eg, excessive vomiting, aspiration of gastric contents with a tube)
- persons suffering from lung diseases (for example, pneumonia, bronchial asthma)
- persons with impaired functioning of the adrenal glands.
Diagnostics
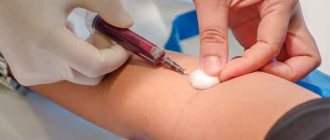
Laboratory research methods are of key importance for the diagnosis and correction of alkalosis. Tests are carried out to determine the blood pH level, its gas composition, electrolyte concentrations and other vital parameters.
Laboratory research:
- Determination of blood pH. The determination of this parameter is based on identifying the concentration of hydrogen ions in arterial blood. With alkalosis, this parameter will be reduced and the pH level will be higher than 7.44.
- Determination of blood gas composition. Blood gas composition is determined using gas analyzers. These devices also allow you to set the blood pH. With alkalosis, the concentration of carbon dioxide in the blood will be reduced.
- General blood analysis. This analysis allows you to evaluate the main blood parameters: the number of red blood cells, hemoglobin, leukocytes, platelets, hematocrit number (the ratio of the number of blood cells to its liquid part). This is of great importance for establishing the causes of alkalosis. For example, one of the possible causes of respiratory alkalosis may be severe anemia, in which a decrease in hemoglobin, red blood cells and hematocrit number will be observed.
- General urine analysis with microscopy. This analysis allows us to identify the basic physical and chemical properties, establish the pH level of urine, and determine the presence of pathological and physiological metabolic products.
- Potassium, sodium, chlorine in serum. Potassium, sodium, chlorine are the main electrolytes in human blood, which are necessary for moving substances inside cells and removing metabolic products from them, maintaining acid-base balance, and carrying out other processes in the body. With alkalosis, significant changes in water and electrolyte balance can occur, which require constant monitoring and correction.
- Aldosterone. Aldosterone is an adrenal hormone that promotes the retention of sodium and water in the body and the excretion of potassium by the kidneys. One of the causes of metabolic alkalosis may be an increased level of this hormone.
- Cortisol. Cortisol is a hormone produced by the adrenal glands. It is involved in the regulation of the metabolism of proteins, fats and carbohydrates in the body, the formation of the body's adaptive response to stress. Determination of the level of this hormone may be required if Cushing's syndrome is suspected, in which alkalosis may occur.
- Alanine aminotransferase (ALT). An enzyme that is present in many cells of the body, but is found in greater quantities in liver cells. When liver cells are damaged, the level of this enzyme in the blood increases significantly. Liver failure may be one of the causes of alkalosis.
Research:
- Radiography. Chest x-rays can identify pathologies in the lungs that may be the cause of respiratory alkalosis (for example, pneumonia).
- Ultrasound examination (ultrasound). This study is based on the properties of ultrasound. The method makes it possible to visualize internal organs, identify changes in their structure, size, and allows you to diagnose the presence of space-occupying formations (for example, cysts, tumors), which is of great importance for identifying the causes of changes in the acid-base balance in the body.
- Computed tomography (CT) and magnetic resonance imaging (MRI). These studies have different operating principles. The computed tomography method is based on the ability of X-ray radiation to pass through tissues of varying densities. Images of human internal structures during MRI are formed as a result of the action of a strong magnetic field on the tissue, followed by registration and computer processing of the received signals.
These techniques make it possible to obtain highly informative layer-by-layer images of internal organs, which is very important in diagnosing the causes of various conditions, including alkalosis (for example, identifying tumors of the adrenal glands, brain).
Treatment
Treatment of alkalosis consists of treating the underlying disease or eliminating other causes that led to the development of this condition. With metabolic alkalosis, there is often a need to correct not only blood pH, but also disturbances in water and electrolyte balance. For this purpose, intravenous infusions of special solutions are performed.
To treat respiratory alkalosis, inhalation of gas mixtures containing carbon dioxide can be used.
Prevention
There is no specific prevention of alkalosis, since this condition can be a consequence of various pathological processes occurring in the body.
Recommended tests
- Blood pH determination
- Determination of blood gas composition
- General blood analysis
- General urine analysis with microscopy
- Potassium, sodium, chlorine in serum
- Aldosterone
- Cortisol
- Alanine aminotransferase (ALT).
Causes
The causes of alkalosis vary in their etiology:
- gas (respiratory) - the reasons lie in hyperventilation of the lungs, which leads to excessive release of CO2 and a drop in partial pressure, most often this pathological condition is associated with disturbances in respiratory rate and organic lesions in the brain (with encephalitis , tumors, etc.), thromboembolism of the pulmonary arteries , pulmonary pathology, hysterical shortness of breath (so-called dog breathing ), with an increase in body temperature and the effect on the respiratory center of various toxic agents, such as microbial toxins, as well as iatrogenics - mechanical ventilation without proper control of the gas composition of the circulating blood;
- non-gas excretory , exogenous and metabolic, while excretory alkalosis is characterized by disturbances in the acid-base balance as a result of large losses of gastric juice (caused by gastric fistulas or uncontrollable vomiting), taking diuretics (especially mercury), increased sweating, as a result of certain kidney diseases, endocrine disorders that initiate excessive sodium retention in the body;
- non-gas exogenous - most often a manifestation of excessive administration of sodium bicarbonate in cases of correction of manifestations of metabolic acidosis or neutralization of an increased concentration of hydrochloric acid in gastric juice, in addition, the development of moderate compensated alkalosis can occur with prolonged consumption of food with a high content of bases;
- metabolic endogenous non-gas etiology alkalosis is characteristic of such pathological conditions associated with disturbances of electrolyte metabolism, such as hemolysis in the postoperative period after undergoing extensive surgical interventions, with rickets and in cases of hereditary disorders of the regulation of electrolyte interactions.
With the mixed nature of alkalosis, gas and non-gas mechanisms are combined, which usually occur against the background of various brain injuries, accompanied by hypoxia, shortness of breath and severe vomiting of gastric juice.
Treatment
A prerequisite is the elimination of the primary cause of alkalosis. It all depends on the specific provoking factor.
- Vomiting requires the prescription of specific drugs. Metoclopramide, Cerucal.
- Sorbents for intense diarrhea. Be it activated carbon or other medications specifically designed for this purpose.
- Neurotic manifestations with uncontrolled hyperventilation require elimination with the help of sedatives (Diazepam). In complex clinical cases, if the patient is in a psychotic state, the use of antipsychotics will be required. However, this is a relatively rare situation.
As for eliminating the symptoms themselves, other medications are used.
- Panangin, Asparkam. Means for normalizing potassium levels in the body. This is a must. Read about other potassium supplements in this article.
- It is possible to use gentle potassium-sparing diuretics: Veroshpiron, Spironolactone and the like. This group of drugs is described in detail here.
- As a measure for the treatment of alkalosis, Darrow's solution and electrolytic salt mixtures are used.
In the first days after recovery from an acute condition, it is necessary to follow a gentle diet. You need to take as little acid-containing foods as possible.
This includes, for example, fermented milk components, fresh fruits, and vegetables. As support, it is possible to prescribe vitamin-mineral complexes and ascorbic acid.
Treatment of alkalosis in the early stages is carried out in intensive care conditions. After 1-2 days, the person, with a successful course and good dynamics, is sent to the general ward. He should remain under supervision for several more days.
Symptoms of alkalosis
The symptom complex of gas alkalosis includes:
- agitation, anxiety, dizziness, fainting, paresthesia of the face and limbs, which is associated with diffuse cerebral ischemia;
- rapid breathing;
- fatigue, decreased concentration and memory;
- pallor of the skin with possible gray diffuse cyanosis - most often in cases of concomitant hypoxemia ;
- increased diuresis leads to dehydration, as well as seizures due to hypocalcemia .
Important! The clinical picture can vary significantly depending on the severity of alkalosis and can cause a slight feeling of anxiety or quickly turn into lethargy, cause a convulsive syndrome, an increase in cardiac output can turn into serious dysfunction of the heart.
Metabolic alkalosis is characterized by the development of other clinical manifestations and may be compensated or transient, including symptoms such as:
- respiratory depression or transition to the superficial type;
- the occurrence of swelling;
- progressive weakness, fatigue, constant feelings of thirst and anorexia;
- headaches with minor hyperkinesis of the muscles of the face and limbs;
- dry skin, decreased tissue turgor.
In more severe conditions, tachycardia or embryocardia . Apathy and lethargy are replaced by drowsiness , which can ultimately lead to a coma.
Diagnostics
The examination is carried out in a hospital setting. The specialized specialist is a hematologist. In most situations you need to act quickly, since there is no time for long-term activities.
An approximate list of ways to assess the condition is as follows:
- Oral interview with a person. It is important to identify all health complaints. This will allow us to draw a picture and put forward basic hypotheses regarding the deviation.
- Anamnesis collection. Previous violations, currently ongoing processes, circumstances of the beginning of the proposed change.
- General blood analysis. It is important to identify baseline indicators. Carried out urgently.
- Electrocardiography is required. The heart suffers first, so it needs to be checked. There are no typical signs of alkalosis, but against the background of the disorder, deviations from the conduction system are observed. First, tachycardia, then the opposite phenomenon with a drop in heart rate and, accordingly, cardiac output.
- Other laboratory tests are ordered if there is time.
Despite the catastrophic limitation of time, it is important to make the correct diagnosis. Otherwise, the effect of help will be zero or, worse, wrong measures will cost a person his life.
In children
The pathogenesis of alkalosis in children is no different from that in adults. However, due to the lability of metabolism and acid-base balance, alkalosis becomes a fairly common manifestation of various pathological conditions and diseases. For example, pyloric stenosis , intestinal obstruction and birth injuries in newborns. Alkalosis in children can be caused by a toxic syndrome that develops in cases of acute respiratory viral infections, traumatic brain injuries , pneumonia , hyperthermia , encephalitis , meningitis , brain tumors and psychogenic reactions.
Metabolic alkalosis is one of the symptoms of Bartter's syndrome or, in other words, hyperreninemic haperaldosteronism, which develops with a hereditary defect in the renal tubular system and disruption of the processes of active transport of chlorine ion. The onset occurs in the first year of life in the form of periodic vomiting, increased body temperature and delayed physical development. Polyuria , polydipsia , hypochloremia , hapokalemia , increased aldosterone and renin are also observed .
Diet for alkalosis
Potassium diet
- Efficacy: the therapeutic effect is achieved after 5 days
- Time frame: 10 days
- Cost of products: 1100-1200 rubles per week
One of the most important aspects of recovery from alkalosis is maintaining a strict diet rich in potassium and sodium. It is important that your diet includes a lot of different fruits and vegetables - raw or steamed, in the form of salads or smoothies. We must not forget about the sufficient amount of proteins that can be obtained from eggs and dietary meats.
Fresh milk and various diuretic products, such as coffee or watermelon, as well as canned goods, pickles, butter, alcohol and carbonated drinks are usually prohibited.