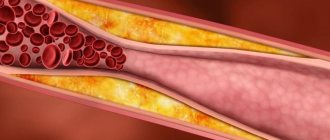
An increase in blood cholesterol levels and the initial stage of atherosclerosis are direct indications for starting therapy. The basis of all medications used in the course of treatment are statins. They reduce the amount of cholesterol produced in the body and significantly improve the patient’s well-being.
One of these drugs is Livazo 2 mg. This medicine is also available in active ingredient quantities of 1 mg and 4 mg, and the specific dosage prescribed depends on the severity of the disease and is at the discretion of the attending physician.
Active substance and indications for use
The active ingredient of the drug Livazo is pitavastatin - this component is a lipid-lowering agent designed to reduce the production of its own “bad” cholesterol in the human body. Livazo is a prominent representative of HMG-CoA reductase inhibitors (statins); this group of drugs is prescribed to almost all patients with high cholesterol in the blood.
Indications for use of Livazo:
- primary hypercholesterolemia (increased cholesterol levels in the blood, recorded for the first time, without linking the pathology to concomitant diseases);
- hereditary hypercholesterolemia (to reduce low-density lipoproteins in the blood).
This drug is also prescribed in cases where it is not possible to reduce cholesterol in patients on a diet or performing therapeutic exercises.
Livazo is produced by the manufacturer in three dosages:
- 1 mg – contains 1.045 mg of pitavastatin calcium (corresponding to 1 mg of pitavastatin);
- 2 mg – includes 2.090 mg pitavastatin calcium (corresponding to 2 mg pitavastatin);
- 4 mg – pitavastatin calcium content 4.180 mg (equivalent to 4 mg pitavastatin).
Tablets in all three dosages are film-coated, they are painted white, have a round shape, on one side there is an engraving of KS, and on the other - the volume of the active substance, indicated by the numbers 1,2,3 (respectively 1 mg, 2 mg, 4 mg ).
According to the official instructions for use of Livazo from the manufacturer, the tablets should be taken orally without chewing first and without coordinating the time of administration with food. The drug is taken once a day, it is advisable to take the tablets at the same time to achieve maximum effect
Livazo tablet p o film 4 mg x28
ATX code: C10AA08 (Pitavastatin) Active substance: pitavastatin (pitavastatin) Rec.INN registered by WHO
Dosage forms
Livazo
Film-coated tablets, 4 mg: 7, 10, 14, 15, 20, 28, 30, 42 or 45 pcs.reg. No.: LP-002855 dated 02/09/15 - Valid Re-registration date: 09/25/15
Film-coated tablets, 1 mg: 7, 14, 15, 28 or 30 pcs.reg. No.: LP-002855 dated 02/09/15 - Valid Re-registration date: 09/25/15
Film-coated tablets, 2 mg: 7, 10, 14, 15, 20, 28, 30, 35, 40, 42, 45, 50, 60, 70, 75 or 100 pcs.reg. No.: LP-002855 dated 02/09/15 - Valid Re-registration date: 09/25/15
Release form, packaging and composition of the drug Livazo
The tablets are white, film-coated, round, biconvex, with a white core on the cross section, engraved “KC” on one side of the tablet, and engraved “1” on the other.
1 tab.
pitavastatin calcium 1.045 mg,
which corresponds to the content of pitavastatin 1 mg
Excipients: lactose monohydrate - 63.085 mg, low-substituted hyprolose - 12.54 mg, hypromellose - 1.33 mg, magnesium aluminum metasilicate - 1.6 mg, magnesium stearate - 0.4 mg.
Film shell composition: Opadry white - 3 mg (hypromellose - 1.98 mg, titanium dioxide - 0.8 mg, triethyl acetate - 0.2 mg, colloidal silicon dioxide - 0.02 mg).
7 pcs. - blisters (1) - cardboard packs. 7 pcs. - blisters (2) - cardboard packs. 14 pcs. - blisters (1) - cardboard packs. 14 pcs. - blisters (2) - cardboard packs. 15 pcs. - blisters (1) - cardboard packs. 15 pcs. - blisters (2) - cardboard packs.
White film-coated tablets, round, biconvex, with a white core on the cross section, engraved “KC” on one side of the tablet, and engraved “2” on the other.
1 tab.
pitavastatin calcium 2.09 mg,
which corresponds to the content of pitavastatin 2 mg
Excipients: lactose monohydrate - 126.17 mg, low-substituted hyprolose - 25.08 mg, hypromellose - 2.66 mg, magnesium aluminum metasilicate - 3.2 mg, magnesium stearate - 0.8 mg.
Film shell composition: Opadry white - 5 mg (hypromellose - 3.3065 mg, titanium dioxide - 1.338 mg, triethyl acetate - 0.3305 mg, colloidal silicon dioxide - 0.025 mg).
7 pcs. - blisters (1) - cardboard packs. 7 pcs. - blisters (2) - cardboard packs. 7 pcs. - blisters (3) - cardboard packs. 7 pcs. - blisters (5) - cardboard packs. 10 pieces. - blisters (1) - cardboard packs. 10 pieces. - blisters (2) - cardboard packs. 10 pieces. - blisters (3) - cardboard packs. 10 pieces. - blisters (5) - cardboard packs. 14 pcs. - blisters (1) - cardboard packs. 14 pcs. - blisters (2) - cardboard packs. 14 pcs. - blisters (3) - cardboard packs. 14 pcs. - blisters (5) - cardboard packs. 15 pcs. - blisters (1) - cardboard packs. 15 pcs. - blisters (2) - cardboard packs. 15 pcs. - blisters (3) - cardboard packs. 15 pcs. - blisters (5) - cardboard packs. 20 pcs. - blisters (1) - cardboard packs. 20 pcs. - blisters (2) - cardboard packs. 20 pcs. - blisters (3) - cardboard packs. 20 pcs. - blisters (5) - cardboard packs.
The tablets are white, film-coated, round, biconvex, with a white core on the cross section, on one side of the tablet there is an engraving “KC”, on the other there is an engraving “4”.
1 tab.
pitavastatin calcium 4.18 mg,
which corresponds to the content of pitavastatin 4 mg
Excipients: lactose monohydrate - 252.34 mg, low-substituted hyprolose - 50.16 mg, hypromellose - 5.32 mg, magnesium aluminometasilicate - 6.4 mg, magnesium stearate - 1.6 mg.
Film shell composition: Opadry white - 9 mg (hypromellose - 5.952 mg, titanium dioxide - 2.409 mg, triethyl acetate - 0.594 mg, colloidal silicon dioxide - 0.045 mg).
7 pcs. - blisters (1) - cardboard packs. 7 pcs. - blisters (2) - cardboard packs. 7 pcs. - blisters (3) - cardboard packs. 10 pieces. - blisters (1) - cardboard packs. 10 pieces. - blisters (2) - cardboard packs. 10 pieces. - blisters (3) - cardboard packs. 14 pcs. - blisters (1) - cardboard packs. 14 pcs. - blisters (2) - cardboard packs. 14 pcs. - blisters (3) - cardboard packs. 15 pcs. - blisters (1) - cardboard packs. 15 pcs. - blisters (2) - cardboard packs. 15 pcs. - blisters (3) - cardboard packs.
Clinical and pharmacological group: Lipid-lowering drug Pharmaco-therapeutic group: Lipid-lowering drug - HMG-CoA reductase inhibitor
pharmachologic effect
Pitavastatin is a competitive inhibitor of HMG-CoA (3-hydroxy-3-methylglutaryl coenzyme A) reductase, an enzyme that catalyzes the initial stage of cholesterol synthesis - the formation of mevalonic acid from HMG-CoA. Since the conversion of HMG-CoA to mevalonic acid is the initial stage of cholesterol synthesis, the use of pitavastatin does not cause the accumulation of potentially toxic sterols in the body. HMG-CoA is easily metabolized to acetyl-CoA, which is involved in many synthesis processes in the body. Livazo has been proven effective in reducing the concentration of total cholesterol (OXC) in blood plasma, LDL cholesterol (LDL-C), VLDL cholesterol (VLDL-C), triglycerides (TG) and apolipoprotein B (Apo-B), as well as increasing the concentration of HDL cholesterol (HDL-C) and apolipoprotein A1 (Apo-A1) (see Table 1).
Table 1. Dose response in patients with primary hypercholesterolemia (adjusted mean percentage change from baseline)
Dose N LDL cholesterol TC* HDL cholesterol TG Aro-B Aro-A1
Placebo 51 -4.0 -1.3 2.5 -2.1 0.3 3.2
1 mg 52 -33.3 -22.8 9.4 -14.8 -24.1 8.5
2 mg 49 -38.2 -26.1 9.0 -17.4 -30.4 5.6
4 mg 50 -46.5 -32.5 8.3 -21.2 -36.1 4.7
*uncorrected
In the treatment of primary hypercholesterolemia and combined (mixed) dyslipidemia, when administered in therapeutic doses, pitavastatin significantly reduced the concentration of LDL cholesterol. TC, non-HDL cholesterol, TG and Apo-B and increased the concentration of HDL cholesterol and Apo-A1. The ratio of TC/HDL cholesterol and Apo-B/Apo-A1 decreased. When using Livazo at a dose of 2 mg, a decrease in LDL cholesterol concentration was achieved by 38-39%, and by 44-45% when using a dose of 4 mg. In most cases, when prescribing a dose of 2 mg, the LDL cholesterol treatment target according to the recommendations of the European Atherosclerosis Society (EAS) was achieved.
When treating patients of the older age group with primary hypercholesterolemia and mixed dyslipidemia (≥65 years), when prescribing a dose of Livazo 1 mg, 2 mg or 4 mg, LDL cholesterol values decreased by 31%, 39% and 44.3%, respectively, the target level established EAS was achieved in 90% of patients.
When treating patients with primary hypercholesterolemia or mixed dyslipidemia in combination with 2 or more cardiovascular risk factors, or mixed dyslipidemia in combination with type 2 diabetes mellitus, approximately 80% of patients achieved EAS LDL cholesterol targets. LDL cholesterol levels in these groups of patients decreased by 44% and 41%, respectively.
With long-term use of Livazo, the target value established by the EAS was maintained due to a constant stable decrease in the concentration of LDL cholesterol (-30.5%), and the concentration of HDL cholesterol consistently increased, with a large increase in the concentration of HDL cholesterol in patients with initially lower values of this indicator (<. 40 mg/dL), from 11.9% after 3 months to 28.9% after 5 years.
Atherosclerosis
In the treatment of patients who underwent percutaneous coronary intervention for acute coronary syndrome under intravascular ultrasound guidance, the administration of pitavastatin at a dose of 4 mg for 8-12 months led to a decrease in the volume of coronary plaques by approximately 17% and was accompanied by a reverse development of vascular wall remodeling (from 113.0 up to 105.4 mm3).
The beneficial effects on mortality and complication rates have not been assessed to date.
Diabetes
In patients with hyperlipidemia and impaired glucose tolerance, when Livazo was prescribed in doses of 1 mg/day or 2 mg/day in addition to recommendations for lifestyle changes, newly diagnosed diabetes mellitus developed less frequently than in patients who did not receive lipid-lowering therapy: 39.9% versus 45.7% over a period of 2.8 years, the risk ratio was 0.82.
Data from a meta-analysis of the safety of pitavastatin in relation to the risk of developing diabetes mellitus demonstrated a neutral effect of Livazo on the risk of developing new cases of diabetes mellitus compared with therapy with other statins.
Pharmacokinetics
Suction
Pitavastatin is rapidly absorbed in the upper gastrointestinal tract, Cmax in blood plasma is achieved within 1 hour after taking the drug. Eating does not affect absorption. Cmax of pitavastatin in blood plasma decreases by 43% when taken together with fatty foods, but AUC remains unchanged. The unchanged drug undergoes enterohepatic recirculation and is well absorbed from the jejunum and ileum. The absolute bioavailability of pitavastatin is 51%.
Distribution
More than 99% of pitavastatin is bound to plasma proteins, mainly albumin and alpha-1 acid glycoprotein. Average Vd 133 l. Pitavastatin actively penetrates into hepatocytes using the transport proteins OATP1B1 and OATP1B3. AUC varies within a 4-fold increase from minimum to maximum values. Pitavastatin is not a substrate for P-glycoprotein.
Metabolism
Plasma contains mainly unchanged pitavastatin. The main metabolite is an inactive lactone, which is formed from the ether-type pitavastatin glucuronide conjugate with the participation of UDP-glucuronosyltransferases (UGT1A3 and 2B7). Cytochrome P450 has a minimal effect on pitavastatin metabolism. The CYP2C9 isoenzyme (and to a lesser extent the CYP2C8 isoenzyme) is involved in the metabolism of pitavastatin to minor metabolites.
Removal
Pitavastatin, unchanged, is rapidly excreted from the liver with bile, but undergoes enterohepatic recirculation, which ensures its long-lasting effect. Less than 5% of pitavastatin is excreted by the kidneys. T1/2 from plasma varies from 5.7 hours (single dose) to 8.9 hours (at steady state), the average clearance is 43.4 l/h after a single oral dose.
Pharmacokinetics in different patient groups
Elderly patients: When evaluating pitavastatn pharmacokinetics in elderly patients over 65 years of age, the AUC of pitavastatin was 1.3 times higher. This did not affect efficacy or safety.
Hepatic impairment: In patients with mild hepatic impairment (Child-Pugh class A), the AUC was 1.6 times higher than in healthy volunteers, while in patients with moderate hepatic impairment (Child-Pugh class B) AUC was 3.9 times higher. In cases of severe liver dysfunction, the use of pitavastatin is contraindicated.
Renal failure: in patients with moderate renal failure and on hemodialysis, an increase in AUC was observed by 1.8 times and 1.7 times, respectively.
Gender differences: there was a 1.6-fold increase in AUC in women compared to men in a study of healthy volunteers, which did not in any way affect the effectiveness and safety of the drug Livazo.
Race: According to the results of pharmacokinetic analysis of data obtained from healthy volunteers of different races (Japanese and Caucasian populations), factors such as gender and age did not affect the pharmacokinetics of pitavastatin.
Indications for the drug Livazo are primary hypercholesterolemia, including heterozygous familial hypercholesterolemia (type IIa hyperlipidemia according to the Fredrickson classification) or mixed hypercholesterolemia (type IIb hyperlipidemia according to the Fredrickson classification), hypertriglyceridemia (type IV hyperlipidemia according to the Fredrickson classification) as an addition to the diet, when diet and others Non-drug treatments (eg, exercise, weight loss) are insufficient. ICD-10 codes
ICD-10 code Indication
E78.0 Pure hypercholesterolemia
E78.1 Pure hyperglyceridemia
E78.2 Mixed hyperlipidemia
Dosage regimen
Inside, tablets must be swallowed whole.
It is preferable to take the tablet at the same time of day, preferably in the evening, in accordance with the circadian rhythm of lipid metabolism. Before starting treatment and during the process, patients should adhere to a cholesterol-lowering diet.
The initial dose of the drug is 1 mg/day once. If necessary, the dose of the drug is increased at intervals of at least 4 weeks to 2 mg/day. The dose should be individualized based on LDL-C concentrations, the goal of treatment, and the patient's response to treatment. Most patients require a dose of 2 mg. The maximum daily dose is 4 mg.
Patients with mild to moderate liver dysfunction: A maximum daily dose of 2 mg is recommended.
Patients with impaired renal function: in case of mild renal impairment (it is advisable to objectively assess this degree with a reflection of CK or GFR), Livazo should be used with caution. Data on the use of a maximum daily dose of 4 mg for renal impairment of any severity are limited, therefore, a maximum daily dose of 4 mg should be prescribed only with careful monitoring of renal function after a gradual dose increase. It is not recommended that patients with severe renal impairment be prescribed a maximum daily dose of 4 mg; it is recommended to consider limiting the maximum daily dose to 2 mg in severe renal impairment.
Elderly patients: no dose adjustment is required.
Side effect
In controlled clinical studies, when taking recommended doses, less than 4% of patients treated with Lnvaso. was excluded from the study due to the development of adverse reactions. The most common was myalgia.
Depending on the frequency of occurrence, the following adverse reactions are distinguished in accordance with the WHO classification: very often (≥10), often (from ≥1/100 to <.1/10), infrequently (from ≥1/1000 to <.1/100 ), rare (≥1/10,000 to <.1/10,000), very rare (<.1/10,000) and frequency unknown (available data do not allow frequency to be determined).
From the hematopoietic organs: infrequently - anemia.
From the side of metabolism: infrequently - anorexia.
Mental disorders: often - insomnia.
From the nervous system: often - headache, infrequently - dizziness, taste disturbance, drowsiness.
From the senses: infrequently - ringing in the ears, rarely - decreased visual acuity.
From the skin: infrequently - itching, soup, rarely - urticaria, erythema.
From the musculoskeletal system: often - myalgia, arthralgia, infrequently - muscle spasms.
From the urinary system: infrequently - pollakiuria.
From the digestive system: often - constipation, diarrhea, dyspepsia, nausea, infrequently - abdominal pain, dry oral mucosa, vomiting, rarely - glossodynia, acute pancreatitis, cholestatic jaundice.
Laboratory indicators: infrequently - increased activity of liver transaminases (AST, ALT), increased CPK activity.
In clinical studies, after taking Livazo, an increase in CK activity was observed 3 times higher than the ULN in 49 patients out of 2800 (1.8%). Levels of 10 times or more ULN with associated muscle symptoms were rare and were observed in only one patient out of 2406 patients receiving 4 mg Livazo (0.04%) in the clinical trial program.
Other: infrequently - asthenia, malaise, increased fatigue, peripheral edema.
Post-marketing experience
A 2-year prospective post-marketing follow-up study was conducted in approximately 20,000 patients in Japan. The vast majority of these patients received pitavastatin 1 or 2 mg, but not 4 mg. In 10.4% of patients, adverse reactions were reported in which a causal relationship with pitavastatin cannot be excluded, and 7.4% of patients discontinued treatment due to the development of adverse reactions. The incidence of myalgia was 1.08%. Most adverse reactions were mild. Over a 2-year period, the incidence of adverse reactions was higher in patients with a history of drug allergies (20.4%) or liver or kidney disease (13.5%).
Adverse reactions and their incidence observed in a prospective post-marketing surveillance study, but not in international controlled clinical studies, with the use of the drug and recommended doses are listed below.
From the liver and biliary tract: rarely - liver dysfunction, liver disease.
From the musculoskeletal system: rarely - myopathy, rhabdomyolysis.
In the post-marketing surveillance study, there were two reports of rhabdomyolysis requiring hospitalization (0.01% of patients).
In addition, there are spontaneous reports of musculoskeletal effects, including myalgia and myopathy, in patients treated with Livazo at all recommended doses. There have also been reports of rhabdomyolysis with and without acute renal failure, incl. about rhabdomyolysis with a fatal outcome.
Spontaneous reports of the following adverse reactions have also been received (frequency based on incidence observed in post-marketing studies).
From the nervous system: infrequently - hypoesthesia.
From the digestive system: rarely - abdominal discomfort.
Adverse events when using other statins:
sleep disturbance, including nightmares, sexual dysfunction, depression, interstitial lung disease, diabetes mellitus: incidence depends on the presence or absence of risk factors (fasting blood glucose ≥5 mmol/l, BMI>30 kg/m2, elevated TG, history of arterial hypertension), increased glycosylated hemoglobin.
Contraindications for use
hypersensitivity to pitavastatin, auxiliary components of the drug and other HMG-CoA reductase inhibitors (statins), severe liver failure (more than 9 points on the Child-Pugh scale) or class C according to the Child-Pugh classification, liver disease and active phase, including persistent increased activity of hepatic transaminases in the blood serum (more than 3 times compared to ULN), myopathy, concomitant use of cyclosporine, pregnancy, breastfeeding, lack of adequate methods of contraception in women of childbearing age, age under 18 years (efficacy and safety have not been established ). lactose intolerance, lactase deficiency or glucose-galactose malabsorption.
Carefully
If there is a risk of developing myopathy/rhabdomyolysis - renal failure, hypothyroidism, personal or family history of hereditary muscle diseases and previous history of muscle toxicity when using other HMG-CoA reductase inhibitors or fibrates, history of liver disease, alcohol abuse, age over 70 years.
Use during pregnancy and breastfeeding
Pregnancy
The use of Livazo during pregnancy is contraindicated. Women of childbearing age should use reliable methods of contraception when treating Livazo. Because Since cholesterol and other products of cholesterol biosynthesis are necessary for fetal development, the potential risk of inhibition of HMG-CoA reductase outweighs the benefit of treatment with the drug during pregnancy. Animal studies have shown that pitavastatin has reproductive toxicity but no teratogenic potential. If the patient is planning a pregnancy, treatment should be discontinued at least one month before conception. If pregnancy occurs while using Livazo. treatment should be stopped immediately.
Breastfeeding period
The use of Livazo during breastfeeding is contraindicated. Pitavastatin is excreted in the milk of lactating rats. There are no data on the excretion of pitavastatin in breast milk. If it is necessary to use the drug Livazo during lactation, breastfeeding should be stopped.
Use for liver dysfunction
For patients with mild to moderate liver dysfunction, a maximum daily dose of 2 mg is recommended.
It is prohibited to use the drug with severe liver failure (more than 9 points on the Child-Pyot scale) or class C according to the Child-Pyot classification, liver diseases in the active phase, including a persistent increase in the activity of “liver” granamnase in the blood serum (more than 3 times compared to the upper limit of normal (ULN)).
Use for renal impairment
The drug should be used with caution in case of renal failure. Data on the use of a maximum daily dose of 4 mg for renal impairment of any severity are limited, therefore, a maximum daily dose of 4 mg should be prescribed only with careful monitoring of renal function after a gradual dose increase. It is not recommended that patients with severe renal impairment be prescribed a maximum daily dose of 4 mg; it is recommended to consider limiting the maximum daily dose to 2 mg in severe renal impairment.
Use in children The use of the drug in patients under the age of 18 years is prohibited (since the effectiveness and safety have not been established).
Use in elderly patients: Elderly patients do not require dose adjustment.
special instructions
Effect on muscle tissue
As with other HMG-CoA reductase inhibitors (statins), there is a possibility of developing myalgia, myopathy and, in rare cases, rhabdomyolysis. Patients should be warned to report any muscle symptoms. CPK activity should be determined in any patient reporting muscle pain, muscle tenderness or weakness, especially if accompanied by malaise or fever.
CPK activity should not be determined after physical exercise or in the presence of any other possible reasons for the increase in CPK that may distort the result. If CPK activity increases (5 times higher than ULN), a control analysis should be performed within 5-7 days.
Very rare cases of immune-mediated necrotizing myopathy (IONM) have been reported during treatment or when discontinuing statins. IONM clinically manifests itself in the form of persistent weakness of the proximal muscles and increased CPK activity in the blood serum, which persist despite the abolition of statins.
Before treatment
Like all statins, Livazo should be prescribed with caution to patients with predisposing factors for the development of rhabdomyolysis. CPK activity should be determined to establish a reference baseline value in the following cases:
renal failure, hypothyroidism, personal or family history of hereditary muscle diseases, previous history of muscle toxicity during treatment with fibrates or other statins, history of liver disease or alcohol abuse, elderly patients (over 70 years of age) with other predisposing risk factors for rhabdomyolysis.
In such cases, clinical monitoring is recommended and the risk of treatment should be considered in relation to the potential benefit. Treatment with Livazo cannot be started if CK levels are 5 times higher than the ULN.
During the flow
The patient should be advised to immediately report muscle pain, weakness or cramps to the doctor. CPK activity should be determined, and treatment should be stopped if CPK activity is elevated (5 times the ULN). Consideration should be given to discontinuing treatment if severe muscle symptoms occur, even if CPK activity does not exceed 5 times the ULN. When symptoms resolve and CPK activity returns to normal, re-prescribing Livazo at a dose of 1 mg may be considered, subject to careful monitoring.
Effects on the liver
Like all statins, Livazo should be prescribed with caution to patients with a history of liver disease or to patients who regularly drink excessive amounts of alcohol. Before starting treatment with Livazo and then periodically during treatment, liver function tests should be performed. Patients with a persistent increase in the activity of liver transaminases (ALT and AST), exceeding ULN by 3 times, should stop treatment with Livazo.
Effects on the kidneys
Livazo should be administered with caution to patients with moderate or severe renal impairment. The dose should be increased only with careful monitoring. The 4 mg dose is not recommended for patients with severe renal impairment.
Diabetes
Some evidence suggests that statin
How the drug works
The active substance of the drug Livazo pitavastatin slows down the rate of production of HMG-CoA reductase, thereby reducing the amount of cholesterol produced by liver tissue. As a result, receptors that produce low-density lipoproteins in the liver are activated, and the amount of total cholesterol in the blood plasma decreases.
Pitavastatin is well absorbed by intestinal tissues, and its bioavailability is 51%. After taking a tablet in a dosage of 1, 2 or 4 mg, the maximum concentration of the substance in the blood plasma is observed after 60 minutes. The absorption efficiency of Livazo is not associated with food intake. According to medical statistics, when pitavastatin was taken orally and at the same time consumed fatty foods, the concentration of the substance in the blood decreased to 43%.
No ads 1
Pharmacological properties
Pharmacodynamic parameters.
pitavastatin is a synthetic lipid-lowering agent that controls cholesterol (c) synthesis by competitively inhibiting the liver enzyme 3-hydroxy-3-methylglutaryl coenzyme a (hmg-coa) reductase. As a result, there is a compensatory increase in the expression of LDL receptors, which contributes to increased LDL catabolism. The structure of pitavastatin, different from other representatives of this group, determines its high affinity for HMG-CoA reductase and a pronounced effect on the level of LDL cholesterol and HDL cholesterol in the blood plasma.
Livazo reduces elevated levels of LDL cholesterol, total cholesterol, TG, apolipoprotein (Apo) B, as well as the TG/HDL and Apo-B/Apo-A1 ratio and increases the content of HDL cholesterol and Apo-A1.
The magnitude of the reduction in LDL cholesterol levels with pitavastatin (2 and 4 mg) is comparable to that with atorvastatin (10 and 20 mg) and simvastatin (20 and 40 mg).
Pitavastatin also increases the plasma level of adiponectin, a protein that has anti-atherosclerotic and anti-inflammatory properties, promotes regression of atherosclerotic plaque (by about 17%).
Pharmacokinetic parameters. Absorption of pitavastatin occurs primarily in the small intestine. Eating does not affect this process. Bioavailability is 51%; time to reach Cmax in blood plasma - 1 hour.
More than 99% of pitavastatin is bound to plasma proteins, mainly albumin and α1-acid glycoprotein.
Pitavastatin is primarily metabolized in the liver. It undergoes glucuronidation by uridine 5-diphosphate glucuronyltransferases (UGT1A3 and UGT2B7) to form the major circulating metabolite, pitavastatin lactone.
The cytochrome P450 system is practically not involved in the metabolism of pitavastatin. The effect is exerted by CYP 2C9 and to a lesser extent by CYP 2C8.
T½ from blood plasma at steady state is almost 9 hours.
It is excreted from the body mainly in feces (79%) and 15% in urine.
The AUC of pitavastatin increases in females, as well as in patients over 65 years of age, which does not affect the safety and effectiveness of the drug.
The content of pitavastatin in the blood plasma 1 hour after administration is dose dependent and has an inverse relationship with body weight, which may indicate higher concentrations of the substance in children.
In the study, the pharmacokinetic parameters of pitavastatin in healthy volunteers of different races (Mongoloids and Caucasians) were similar.
Instructions for use
Therapy with statin drugs requires mandatory adherence to a diet with a reduced amount of plant-based fats in the diet. When using Livazo, preference should be given to plant foods, cereals, grains, vegetables and fruits.
It is necessary to give up table salt and sugar or reduce the amount of their consumption. Prohibited foods are fatty meats and fish, bread, carbonated drinks, baked goods, spicy, over-salted, smoked foods. The dosage of pitavastatin per day required for a particular patient is selected in accordance with the test results.
The patient buys Livazo at the pharmacy exclusively in the dose recommended by the attending doctor. Initially, the patient is prescribed tablets at a dosage of 1 mg, adjusting the recommendation over the next 4 weeks. Practice shows that for most people with elevated blood cholesterol levels, the best option is to take Livazo 2 mg once a day.
Contraindications and side effects
Statins are a group of drugs that have a large number of side effects, which is why their independent use is unacceptable. Patients often complain about the following negative effects of Livazo:
- headache;
- nausea, urge to vomit, disturbances in stool stability (alternating constipation and diarrhea);
- pain in muscles and joints, myalgia;
- drowsiness or, conversely, insomnia;
- slight loss of taste;
- general malaise.
With long-term therapy with statins, some patients experience ringing in the ears, swelling on the body, and allergic-type rashes on the skin. The level of hemoglobin in the blood may decrease and the volume of transaminases may increase.
The rarest side effects of Livazo include impaired liver function, the development of acute pancreatitis, significant deterioration in memory and visual function, and a tendency to depression and psychosis. In isolated cases, patients taking statins have experienced nightmares, decreased libido, and loss of sexual function.
You should not take statin drugs, including Livazo, if you have the following health problems:
Doctor Myasnikov’s opinion on the benefits and harms of statins
- liver diseases occurring in acute form or their chronic forms in the acute stage;
- special sensitivity to the drug group statins and their active ingredients;
- galactose intolerance (including those having a hereditary etiology);
- malabsorption syndrome;
- simultaneous use of drugs from the cephalosporin group;
- myopathy.
Statins are not prescribed to patients under 18 years of age, as well as to women who are pregnant or breastfeeding a newborn child. If Livazo is recommended for use by a woman of childbearing age, she must take oral contraceptives so that an unexpected pregnancy does not occur during statin therapy.
No ads 2
Contraindications
- Hypersensitivity to the active substance or any of the auxiliary ingredients of the drug, as well as to other statins;
- severe liver dysfunction; active stage of liver pathology or a persistent increase of 3 values of the upper limit of normal (ULN) of aminotransferase activity in the blood serum;
- myopathy;
- combined use with cyclosporine;
- pregnancy;
- breastfeeding period.
Possible effects on the body
Speaking about the use of statin drugs, including Livazo, to lower blood cholesterol levels, one cannot fail to mention the effect they have on the human body. It has been established that taking Livazo does not require giving up driving a car or controlling moving mechanisms.
Before prescribing statins, specialists need to make sure that the patient does not have a tendency to rhabdomyolysis. This is a syndrome of extreme myopathy, when muscle tissue cells are destroyed, the level of myoglobin and creatine kinase in the blood rises, as a result of which signs of kidney failure quickly increase.
To avoid this, first monitor the level of creatine kinase in the blood and determine the presence of certain problems:
- dysfunction of the thyroid gland;
- problems with muscle tissue of a personal or hereditary nature;
- taking fibrates or statins resulting in muscle intoxication;
- tendency to alcoholism and diseases of the liver system;
- age over 65–70 years (elderly patients are more prone to rhabdomyolysis).
Livazo is a representative of statins, which reduces the level of “bad” cholesterol in the blood
During treatment with Livazo, if severe muscle symptoms occur, the drug should be discontinued. When creatine kinase levels return to normal, you can resume using Livazo, but under strict medical supervision and at an initial dose of 1 mg per day.
The effect of statins on certain organs and systems:
- liver – if you are prone to alcoholism or have existing disorders in the liver, after prescribing statins, it is necessary to monitor the indicators of the hepatic system (in particular, plasma transaminases);
- kidneys - in the presence of renal failure (moderate and severe) during treatment with Livazo, it is necessary to monitor the functioning of organs. Patients with this disease are not prescribed the drug at a dosage of 4 mg;
- lungs - in the case of long-term therapy with statins, the development of interstitial diseases of the pulmonary system, accompanied by shortness of breath, dry cough, sudden weight loss and fever, was sometimes observed.
If acute signs of liver or kidney failure, as well as interstitial lung diseases, develop, you should immediately consult a doctor and stop taking Livazo. Over time, after the disappearance of pathological symptoms, treatment can be continued, but with a dosage adjustment of statins.
No ads 3
Cross interaction
During the period of use of pitavastatin, it is necessary to take into account the use of drugs that were previously recommended to the patient to eliminate signs of concomitant diseases. Below is a list of pharmacological groups with which you must be careful when interacting with Livazo:
- Cyclosporine - simultaneous use of statins is prohibited due to a sharp increase in AUC;
- Erythromycin and other macrolide antibiotics give the same reaction as taking statins while taking Cyclosporine, so they cannot be used;
- fibrates – simultaneous therapy with statins leads to the development of myopathy and rhabdomyolysis with severe muscle damage (requires selection of the correct dose and caution);
- Niacin and fusidic acid also lead to the formation of gross disorders of muscle tissue;
- Rifampicin and protease inhibitors - reduce the absorption of pitavastatin by liver tissue;
- Warfarin - when taken simultaneously with Livazo 4 mg, monitoring of prothrombin time is necessary.
Drugs such as Digoxin, inhibitors of CYP3A4, OATP1B1 do not affect the concentration of pitavastatin in the blood, so their use simultaneously with Livazo is allowed by doctors.
Interactions
Do not combine with cyclosporine.
In the case of using macrolide antibiotics, as well as fusidic acid, Livazo therapy should be discontinued for this period.
Use with caution in combination with vitamin B3 and fibric acid derivatives.
The AUC of pitavastatin increases when used concomitantly with rifampicin and changes slightly when combined with protease inhibitors.
In case of combined use with warfarin, monitoring of the hemostatic system (prothrombin time or INR) is required.
There is no interaction with the P-glycoprotein substrate digoxin and no clinically significant changes in the content of pitavastatin in the blood plasma when combined with CYP3A4 inhibitors.
Analogues of the drug
If for some reason it is impossible to prescribe pitavastatin to a patient, you can select analogues of Livazo, which are also in the statin group:
- Crestor is a drug belonging to the statin group, based on the active substance rosuvastine (available in dosages of 10, 20 and 40 mg);
- Simvastatin – tablets based on simvastatin, available in the form of 10 and 20 mg dosages;
- Lescol is a drug whose active component is fluvastatin sodium. Sold in the form of capsules containing 21.06 and 42.12 mg of active ingredient;
- Lipobay - tablets consisting of cerivastatin sodium in a dose of 0.2 or 0.4 mg;
- Pravastatin – includes the same name (pravastatin) as the main substance;
- Liprimar is a product containing atorvastatin in dosages of 10, 20, 40 and 80 mg.
Preparations with a similar composition that can replace Livazo
Each of the listed drugs has its own contraindications and side effects, which is why you cannot independently replace Livazo with analogues. Only a specialist can evaluate the medications already taken by the patient, knows the medical history and examination results, and is able to recommend one or another substitute.
Reviews
It should be noted that the majority of patients who were prescribed the drug Livazo, and doctors who work with these tablets, leave only positive reviews. Doctors prescribe pitavastatin due to the quickly onset effect of administration and the good tolerability of Livazo in most patients. Naturally, before prescribing tablets, a complete examination is necessary, and during therapy, monitoring of body functions and blood counts is necessary.
Svetlana, 54 years old: When the doctor prescribed me Livazo 2 mg tablets, I started reading information about them on medical websites. I was afraid of a lot of side effects. But in fact, my body tolerated the pills well, especially since I need to take them once a day. At the same time, I followed a diet that I still maintain today. After 2 months, my blood cholesterol levels dropped and I began to feel much better.
Olga, 48 years old: Livazo tablets were prescribed to me by my doctor after my blood cholesterol rose sharply due to excess weight. At the same time, I was prescribed therapeutic exercise sessions, a diet and walks in the evenings. Now I try to eat healthy food, provide myself with physical activity, and take Livazo 2 mg once a day. The last time I took tests, the doctor said that there were significant improvements.
Vasily, 56 years old: I really like Livazo, it gives results within a month. You feel much better, and the doctor says that tests confirm progress in therapy. One disadvantage of the tablets is their high cost for me. But my sister, who lives in Germany, says that Livazo is a very effective drug; it has been widespread among them for 10–15 years. Therefore, I follow the doctor’s orders; health is still more expensive than pills.
Note!
Description of the drug Livazo table. p/o 1 mg No. 30 on this page is a simplified author’s version of the apteka911 website, created on the basis of the instructions for use.
Before purchasing or using the drug, you should consult your doctor and read the manufacturer's original instructions (attached to each package of the drug). Information about the drug is provided for informational purposes only and should not be used as a guide to self-medication. Only a doctor can decide to prescribe the drug, as well as determine the dose and methods of its use.