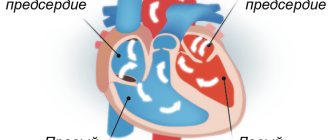
Definition
Heart flutter is a generalized colloquial definition of one of two pathological conditions.
- Flutter of the ventricles of the heart.
- Atrial flutter.
Ventricular flutter is a life-threatening condition in which the heart is unable to pump blood through its vessels. It is accompanied by loss of consciousness, rapidly increasing symptoms of tissue hypoxia, and without immediate resuscitation, it leads to death within a few minutes.
Therefore, in general practice, heart flutter is most often understood as atrial flutter.
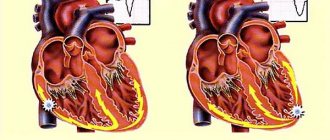
Atrial flutter (AFL) is a cardiac arrhythmia characterized by a rate of 240–400 beats per minute, usually with some degree of atrioventricular (AV) node conduction block. In the most common form of atrial flutter (typical AFL), electrocardiography (ECG) shows a negative sawtooth pattern in leads II, III, and aVF.
Typical (or classic) AFL involves a single reentrant circuit with activation of a circle in the right atrium around the tricuspid annulus. The front of an electric wave most often propagates counterclockwise. Atypical TP occurs according to a different pattern. It may involve the right or left atrium.
TP is associated with a variety of heart diseases. In most studies, approximately 60% of patients with atrial flutter have coronary artery disease (CAD) or hypertension; 30% do not have heart disease. Unusual forms of atrial flutter were noted during long-term follow-up in 26% of patients with surgical correction of congenital heart defects.
Symptoms in patients with AFL usually reflect decreased cardiac output as a result of increased ventricular rate. The most common symptom is rapid heartbeat. Other symptoms include fatigue, shortness of breath and chest pain. An ECG is important for making a diagnosis. Transthoracic echocardiography (TTE) remains the initial imaging modality of choice for the evaluation of AFL.
In this condition, it is very important to control the ventricular response rate or return the patient to sinus rhythm. First, immediate electrical cardioversion is considered for hemodynamically unstable patients. Catheter ablation remains the most appropriate therapy for patients with typical AFL if they meet the contraindication criteria.
Atrial flutter is similar in many ways to fibrillation (underlying disease, pathological factors, complications and treatment). Some patients even experience both flutter and atrial fibrillation. However, the underlying mechanism of AFL makes this arrhythmia amenable to treatment with percutaneous catheters.
What is the diagnosis
The described pathological process in cardiology is called atrial fibrillation or atrial fibrillation. This deviation is classified as tachyarrhythmia - pathological conditions in which the heart rate is significantly higher than normal.
The frequency of nerve impulses responsible for the contraction of certain parts of the heart reaches from 350 to 700 every minute. This intense pace precludes the possibility of a coordinated reduction.
Classification and mechanism of occurrence of disorders
In humans, the most common form of atrial flutter (typical) involves a single reentrant circuit with cycle activation in the right atrium around the tricuspid annulus (most often in a counterclockwise direction) with a region of slow conduction located between the tricuspid valve, the annulus, and the coronary sinus ostium (sub-Eustachian isthmus).
Typical counterclockwise atrial flutter has caudocranial activation (counterclockwise around the tricuspid annulus when viewed on a left anterior oblique fluoroscopic image) of the interatrial septum.
A typical AFL may also have a reverse activation sequence (clockwise around the tricuspid annulus). This option is much less common. When electrical activity moves clockwise, the electrocardiogram (ECG) will show positive flutter waves in leads II, III, and aVF, and they may appear somewhat sinusoidal. This arrhythmia is still considered to be a typical isthmus-dependent flutter. It is usually called reverse typical TP.
Atypical AFL is less studied and electroanatomically characterized. It can happen:
- from the right atrium as a result of surgical scars;
- from the left atrium, in particular from the pulmonary veins (focal entry site);
- from the mitral ring.
Left atrial flutter is a common complication after linear ablation procedures (for atrial fibrillation).
Causes
TP is associated with a variety of heart diseases. In most studies, approximately 30% of patients with atrial flutter have coronary heart disease, 30% have hypertensive heart disease, and 30% have no underlying heart disease. Rheumatic heart disease, congenital heart disease, pericarditis, and cardiomyopathy can also cause flutter. In rare cases, mitral valve prolapse or acute myocardial infarction are associated with this condition.
In addition, the following conditions are associated with TP:
- hypoxia;
- chronic obstructive pulmonary disease;
- pulmonary embolism;
- hyperthyroidism;
- pheochromocytoma;
- electrolyte imbalance;
- alcohol consumption;
- obesity;
- digitalis glycosides;
- myotonic dystrophy in childhood.
AFL may be a consequence of open heart surgery. After cardiac surgery, it can occur as a result of the appearance of natural barriers - incisions in the atria and cardiac scars. Some patients develop atypical left atrial flutter after pulmonary vein isolation procedures for atrial fibrillation.
Although there are no clearly defined genetic conditions that cause atrial flutter, in many cases there is likely a genetic predisposition to its occurrence. Genome-wide association studies in recent years have identified genes associated with atrial flutter.
The PITX2 (paired homeodomain2) gene at the chromosome 4q25 locus is known to play an important role in left-right cardiac asymmetry and has been found to have a strong association with atrial fibrillation and an even stronger association with typical AFL. But there are no clinically available genetic tests that could identify people at increased risk of LT.
Symptoms and accompanying manifestations
Symptoms in patients with AFL usually reflect decreased cardiac output as a result of increased ventricular rate. At the same time, contrary to popular belief, the feeling of “heart fluttering” does not occur. A condition that some patients tend to describe as “heart fluttering” occurs with paroxysmal ventricular tachycardia.
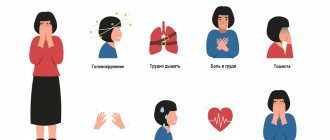
Typical symptoms of TP include:
- dizziness;
- fatigue or poor exercise tolerance;
- slight shortness of breath;
- pre-fainting state.
Less common symptoms include angina, severe shortness of breath, or fainting resulting from poor left ventricular function. With this arrhythmia, thromboembolic phenomena are possible.
In addition, patients may have symptoms of conditions that cause AFL. They may be non-cardiac (such as hyperthyroidism or lung disease) or cardiac.
Often, arrhythmia in these conditions occurs under the influence of provoking factors - alcohol, pneumonia, acute myocardial infarction and surgical procedures. Stimulants such as ginseng, cocaine, ephedra or methamphetamine can also trigger an attack.
For AFL lasting more than 48 hours, anticoagulation with warfarin or transesophageal echocardiography is necessary to exclude left atrial thrombus before cardioversion to sinus rhythm.
The AFL rhythm itself is unstable and usually reverts to either atrial fibrillation or sinus rhythm.
Remaining stable chronic AFL is uncommon, but possible. A history of preexcitation syndrome (Wolf-Parkinson-White) puts the patient at risk for 1:1 flutter waves, which can cause ventricular fibrillation and death.
Patients who are simultaneously diagnosed with new-onset rapid atrial fibrillation or atrial flutter and decreased left ventricular ejection fraction tend to have a high incidence of left atrial appendage thrombus.
What is heart flutter
This definition most often refers to an unpleasant sensation that occurs as a result of excessive heartbeat intensity or deviations in the rhythm of the organ. Normally, when at rest, she practically does not feel her own heartbeat.
Increased heart rate can be associated with numerous factors, and most often occurs due to natural causes. However, the fluttering of the heart, which is accompanied by a pronounced deviation in the frequency of contraction and relaxation processes, is a pathology.
How does fluttering in the chest occur?
To understand how the unpleasant sensation occurs, you need to know the peculiarities of the heart. Normally, the rhythm of this organ is set by the sinus node, which generates nerve impulses that activate contraction of the cardiac atria. When they contract, blood moves into the ventricles, and from there, under the influence of a nerve impulse, is released into the blood vessels.
In case of a violation, a nerve impulse is formed simultaneously in several parts of the atria. Because of this, blood does not have time to enter the atria to transfer it to the ventricles. As a result, the patient experiences a feeling as if the heart is fluttering.
Diagnostics
Electrocardiography (ECG) can provide important information to distinguish “typical” from “atypical” AFL. Transthoracic echocardiography (TTE) is considered the initial diagnostic modality of choice for evaluating atrial flutter.
History and physical examination findings guide laboratory testing. Although hyperthyroidism remains a rare cause of AFL, asymptomatic hyperthyroidism, especially in older patients, may present as fibrillation or AFL and should be excluded during thyroid function testing.
A complete blood count is performed if anemia is suspected or if the patient has a history of recent or current blood loss associated with presenting symptoms.
Chest radiography may be helpful in assessing lung disease and pulmonary vasculature. Chest radiographic findings are usually normal in patients with AFL, but radiographic evidence of pulmonary edema may be noted in subacute cases.
Diagnostic procedures
If the described symptoms occur and tachycardia or arrhythmia appears, you must visit a cardiologist. A comprehensive diagnosis of atrial flutter involves several instrumental and laboratory procedures. Usually, the cause of the disorder cannot be detected immediately, so a comprehensive examination is required.
During the initial examination, the doctor conducts an external examination of the patient, taking anamnesis, palpation and auscultation. This allows you to confirm the presence of arrhythmic abnormalities. Subsequent procedures are necessary to obtain accurate information about the pathology and select optimal treatment methods.
Methods used include:
- Electrocardiography
- Magnetic resonance imaging (including contrast)
- Echocardiography
- Coronary angiography
When making a diagnosis, numerous functional tests and tests are also used to accurately determine the nature of the heart’s work under certain loads. For auxiliary purposes, the patient needs to have blood and urine tested.
Treatment
The general goals of treatment for symptomatic AFL are similar to those for the treatment of atrial fibrillation and include five goals.
- Ventricular rate control
- Restoration of sinus rhythm
- Preventing recurrent episodes or reducing their frequency or duration
- Prevention of thromboembolic complications
- Minimizing side effects from therapy
However, these goals must be selected based on the needs of each patient. In the acute setting of anticipated hemodynamic collapse, immediate electrical cardioversion is considered for hemodynamically unstable patients according to the Advanced Adult Life Support (ACLS) algorithms for the treatment of atrial fibrillation and AFL.
The main difference between atrial fibrillation and atrial flutter is that most cases of AFL can be treated with radiofrequency ablation (RFA). In all available studies, catheter ablation is superior to heart rate and rhythm control strategies with antiarrhythmic drugs.
In Belgium and other European Union countries, catheter ablation has become the first-line treatment for patients with typical atrial flutter in the absence of contraindications. Ablation is usually performed as an elective procedure, but it can also be done for sudden AFL.
Given its high efficacy and low complication rate, RFA is superior to drug therapy. Successful ablation reduces or eliminates the need for long-term use of antiarrhythmic drugs and anticoagulants (unless the patient also has atrial fibrillation).
Drug therapy
As in patients with atrial fibrillation, the decision about the need for post-conversion anticoagulation is made after consideration of each patient's individual thromboembolic and bleeding risks. Data from transesophageal echocardiography (TEE) studies suggest that anticoagulant therapy is recommended after conversion because adnexal flow velocity becomes very low immediately after conversion and is slow to recover.
For episodes of AFL of undetermined duration or more than 48 hours, anticoagulant therapy should be mandatory. Rate monitoring and therapeutic anticoagulation are required at least 4 weeks before cardioversion. If cardioversion is needed earlier, anticoagulant therapy with intravenous (IV) heparin is given as close to the time of cardioversion as possible. Anticoagulation therapy is also required for at least four weeks after cardioversion. If a thrombus is observed or suspected based on TEE results, cardioversion is postponed.
Contraindications to catheter ablation in patients with AFL are a reason to change treatment. Rate and rhythm control strategies are effective in these patients. Due to the risk of arrhythmia, drugs such as ibutilide, sotalol and dofetilide should be prescribed in hospital settings. Complications may occur after transition to sinus rhythm. The risk of proarrhythmia is probably greatest during the first 24 to 48 hours after starting antiarrhythmic drugs.
Preferred medications that slow atrioventricular (AV) node conduction include beta blockers (labetalol, atenolol, metoprolol, propranolol) and calcium channel blockers (eg, verapamil, diltiazem). These medications are used to control ventricular rhythm. They are also used in patients taking Class IA or IC antiarrhythmic drugs (to prevent the rapid ventricular response that may occur when the atrial rate slows).
Consideration of the use of anticoagulants in this patient population (at least until sinus rhythm is maintained) is imperative. Anticoagulant therapy (eg, warfarin) is indicated, especially if AFL lasts more than 48 hours or its onset is unknown.
Patients should be maintained at a therapeutic international normalized ratio (INR) for 3 weeks before conversion and at least 4 weeks after conversion to sinus rhythm.
In patients with chronic AFL, continuous long-term anticoagulant therapy is recommended. Particular caution is required when adding additional medications (including antibiotics) as they may dramatically change the INR in patients taking warfarin.
In patients requiring cardiac surgery, altering the atrial incision and creating a cryothermal lesion similar to the lesion created by radiofrequency catheter ablation may prevent recurrent postoperative arrhythmia.
Prognosis for atrial flutter
It is quite difficult to predict the outcome of the disease, since the nature of the pathology is individual for each patient. In the absence of severe damage to the cardiovascular system and proper antiarrhythmic therapy, the risk of complications is minimized.
This eliminates the possibility of regular relapses. As a rule, repeated attacks occur against the background of age-related changes.
Possible complications
Atrial fibrillation poses a direct threat only in the presence of concomitant heart disease. If there is no concurrent pathology, then the arrhythmic process does not affect the organ. In prolonged forms, due to impaired filling of the ventricles and a decrease in the release of blood into the arteries, heart failure may develop.
Possible complications include:
- IHD
- Ischemic stroke
- Neuralgia
- Atrial thrombosis
- Extensive heart attack
- Regular attacks of angina
The described pathology poses a threat to other organs and systems, which is explained by a decrease in the volume of emitted blood. First of all, the liver, kidneys and lungs are subject to increased stress, which is fraught with additional complications.