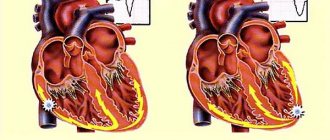
Medical technology has reached such a high level that today surgeons can even perform heart transplants. Only there are very few donor organs, which is why patients who need them are forced to wait a long time for their appearance and sometimes do not live to see this moment. To increase life chances, surgeons often transplant artificial organs into people: engineers have already come up with mechanical versions of ears, eyes, kidneys and other vital body parts. One of the most complex organs in the human body is the heart, which is necessary to supply the organs with blood rich in oxygen and nutrients. The artificial heart already exists and the French company Carmat recently released a new version. It is equipped with biological valves, thanks to which a person is less likely to experience complications, and the device itself is much quieter. This is an important advance in the field of medicine, so let's study the design of an artificial heart in more detail.
Artificial heart from the French company Carmat
Artificial heart: classics and innovations
August 22, 2021 marks the 98th anniversary of the birth of Denton Cooley, the man who first transplanted an artificial heart into a patient, and 49 years since the operation itself. What has changed in cardiac transplantology over the past half century?
What is an “artificial heart”
Today two rather different types of devices are called artificial hearts. Both of them, of course, perform a pumping function, “pumping” blood throughout the human body. However, the first type of machine - the so-called AVK (heart-lung machine) - are large machines used during heart surgery. In fact, they represent a “heart-lung” complex, since they consist of a pump itself, pumping blood, and an oxygenator, supplying this blood with oxygen.
The second type is a cardiac prosthesis, i.e. a device that can perform the functions of the heart after implantation into the human body. It was this type of device that was first transplanted into a patient in 1969 by Denton Cooley - as a temporary measure while the patient waited for a suitable donor heart. Then the patient lived 65 hours after the operation - and this was already a success.
Main problems
There are, of course, many difficulties in such a matter as the design of an artificial organ. But among them two main problems can be identified. The first is energy sources: the heart is inside, and the batteries must be outside so that they can be changed without involving a surgical team.
The wire connecting the battery and the mechanism serves as an excellent “road” for pathogenic microorganisms that cause various types of infections. In addition, the batteries themselves are very large - a whole backpack on the back of the patient, with whom you have to be inseparable.
The second is thrombosis. Even patients who have only one synthetic valve in their natural biological heart must take medications that reduce clotting (warfarin, rivaroxaban) for life. When the entire heart is synthetic, the likelihood of thrombotic complications increases tenfold.
This problem has not yet been completely solved, but there is a compromise solution: artificial hearts eject blood not in bursts, like “natural” ones, but in a continuous continuous stream. In such a flow there are no turbulent turbulences, and blood clots form much less frequently.
Your own battery
It has not yet been possible to get rid of wires going from outside to inside, although there are very interesting ideas in this regard. For example, there was an attempt to organize recharging directly through the patient’s skin. However, the potential difference leads to the development of severe dermatitis. To solve this problem, special protective gels are now being developed (which can simultaneously improve the conductive properties of the skin).
But in order to get rid of a heavy backpack with batteries, the inventors proposed a very elegant solution. The patient puts a special exoprosthesis on his leg (outwardly it is similar to orthoses worn by people with an injured knee), in which a generator is mounted.
While walking, mechanical energy is transformed into electricity, which powers the mechanical heart. We can say that Zhvanetsky, in his old miniature, predicted the appearance of this device - when he proposed attaching a dynamo to the ballerina to generate electricity.
From customer's material
Biotechnology is not lagging behind, in particular, growing organs from patient stem cells. The heart has one advantage over many other organs: it doesn't have many different types of cells. But, on the other hand, the difficulty is that the bulk of the heart cells - contractile cardiomyocytes - must be located in one direction. After all, contracting synchronously, they should cause one powerful movement, and not the picture of “swan, crayfish and pike.”
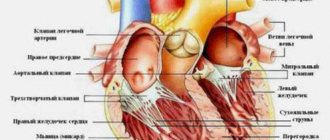
How does the heart work?
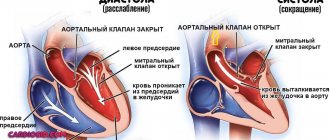
Let me immediately note that we will briefly consider the work of an artificial heart, because this is a very complex organ and an entire book needs to be written to explain all the principles. According to Interesting Engineering, the implant almost completely imitates the work of a real heart. This organ pumps blood by contracting four different chambers: the left atrium, the right atrium, the left ventricle and the right ventricle. In healthy people, one cycle (beat) consists of three short stages:
- atrial filling, in which blood from the large veins fills the atria (0.10 seconds);
- contraction of the ventricles, during which blood is sent to the organs (0.32 seconds);
- break during which the heart rests (0.4 seconds).
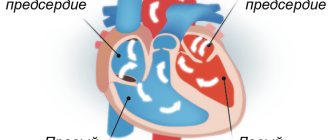
The right ventricle and left atrium are responsible for the pulmonary circulation . In a small circle, oxygen-poor venous blood flows from the right ventricle of the heart through the pulmonary arteries to the lungs and is enriched with oxygen. So from venous it turns into arterial and goes through the pulmonary veins to the left atrium.
Structure of the human heart
The left ventricle and right atrium are responsible for the systemic circulation . This circle is necessary for the blood supply to all organs of the human body, including the lungs.
Systemic and pulmonary circulation of humans
Again, I note that this is a very brief description. If you want to understand exactly how the heart works, it’s better to watch this video. But first, let's look at the artificial heart from Carmat.
Publications in the media
MECHANICAL PROSTHETICS
• Types of mechanical prostheses •• Ball (not currently used): Magovern-Cromie, Smeloff-Cutter, Cross-Jones, AKCh, MKCH •• Disc: Bjork-Shiley, Sorin Monocast, Medtronic-Hall, Lillehei-Kaster, Omniscience , Bicer, MIX, LIX •• Bivalve: St. Jude Medical, Sorin Bi Carbon, Carbomedics, ATS, Duromedics, MedEng.
• Advantage •• Durability—averaging over 30 years (excluding cases of prosthetic infective endocarditis [IE]) •• Exceptions include obturator strut failure in some Bjork–Shiley valve models.
• Disadvantages •• Thrombogenicity (the least with the St. Jude Medical valve) •• The need for constant anticoagulant therapy •• Relative stenosis in disc and ball prostheses •• Post-prosthetic aneurysms in disc prostheses with a small opening angle of the obturator element.
• Anticoagulant therapy •• Begin 2 days after surgery, despite therapy with indirect anticoagulants (INR 3.0–4.5) •• The risk of thromboembolism is high (0.2% of fatal and 2% of non-fatal complications per year) •• Thrombosis after mitral valve replacement occurs much more often due to aortic valve replacement •• Ambiguous attitude towards mechanical valves in the tricuspid position (as a rule, St. Jude Medical prostheses are used) is caused by frequent thrombus formation in the right side of the heart ••• Such thrombus formation is due to apparently a low concentration of PgI2, which has an antithrombotic effect. This Pg is synthesized in the lungs and enters the bloodstream into the left side of the heart •• If thromboembolism caused by the presence of a prosthesis has occurred at least once, then the risk of repeated embolisms is increased •• Constant anticoagulant therapy, even with a moderate increase in INR (up to 3.0) , significantly increases the risk of bleeding.
BIOLOGICAL XENOPROSTHESES (Hancock, Carpentier-Edwards, KemKor) and allografts . In terms of hemodynamic characteristics, both valves are comparable to low-profile mechanical prostheses (bicuspid and disc).
• Advantages •• Thrombogenicity is significantly lower than that of mechanical prostheses •• Anticoagulant therapy is necessary only for 2–3 months after surgery (until endothelialization of the sewing ring occurs).
• Disadvantages •• Short life due to degenerative changes and IE, usually appearing 4-5 years after surgery and further progressing •• Probability of dysfunction 10 years after surgery - 20%, after 15 years - 50% •• Durability of allografts is slightly higher; than other biological prostheses.
• Factors influencing longevity •• Patient age—the likelihood of graft dysfunction is inversely proportional to age at the time of surgery •• Position—the likelihood of graft dysfunction in the mitral position is lower than in the aortic position •• CRF and hypercalcemia in hyperparathyroidism—increases the risk of dysfunction and the rate of its progression •• Pregnancy—shortened service life due to greater hemodynamic load (volume).
Prosthesis selection • Mechanical prostheses •• Aortic position or the need for simultaneous mitral and aortic valve replacement •• IE or prosthesis reimplantation due to IE •• Hypercalcemia •• Renal failure • Biological prostheses •• High risk of thrombosis (see below) •• Contraindications to anticoagulant therapy (hemorrhagic diathesis, repeated gastrointestinal bleeding, alcoholism, unwillingness or inability to follow the treatment regimen), planned pregnancy, age over 65-70 years, TC replacement (even with simultaneous implantation of a mechanical prosthesis in the mitral or aortic position).
Complications • Relative stenosis of the prosthesis (acceptable pressure gradients on the prostheses are indicated in the technical documentation for them) • Obstruction of the prosthesis due to thrombosis, fibrosis, proliferation of vegetations • Valvular (thrombosis of the prosthesis or vegetation) or perivalvular (ring abscess, prosthesis separation) regurgitation • IE • • Prosthetic IE ••• Prevalence - 1–4% per year ••• Early (before 90 days after surgery) and late infective endocarditis can be caused by different pathogens ••• IE is distinguished by its clinical course and complications ••• With infection In mechanical prostheses, damage to the valve itself rarely occurs, but there is a tendency for the biological material covering the sewing ring to be involved in the process. ••• When a biological prosthesis becomes infected, damage to both the leaflets and the valve ring occurs. Vegetations may not be visualized by echocardiography ••• Regurgitation occurs due to destruction or perforation of the leaflets of a biological prosthesis, a mechanical obstruction to the normal movement of a ball or disk created by vegetations •••• Perivalvular regurgitation occurs due to abscess of the valve ring and failure of the sutures ••• Valve obstruction occurs more often due to massive vegetations (caused by fungi, Haemophilus parainfluenzae) •••• Obstruction of a biological prosthesis by vegetations more often occurs in the mitral than in the aortic position ••• Infection of prostheses is relatively difficult to respond to antibiotic therapy, most often re-prosthetics is necessary ••• Treatment of IE - see Infectious endocarditis.
Assessing the condition of the prostheses • On auscultation, opening (usually quieter) and closing clicks (usually louder) of mechanical prostheses are heard; their disappearance or decrease in intensity is a sign of thrombosis • A gentle mesosystolic murmur can be heard over disc prostheses • Biological prostheses do not give a specific auscultatory picture • X-ray examination is currently practically not used specifically to study the condition of prostheses • With EchoCG, the type of prosthesis is determined, the diameter and amplitude are measured opening of the obturator element of the mechanical prosthesis, measure the pressure gradient on the prosthesis and diagnose the presence of pathological flows, vegetations, thrombosis, fibrosis • Transesophageal echocardiography to visualize blood clots and vegetations is carried out if prosthetic dysfunction is suspected or in case of atrial fibrillation/flutter • Invasive diagnostic methods are indicated only in case of difficulties in diagnostics.
Anticoagulant therapy • For non-cardiac operations that are fraught with large blood loss, anticoagulants are discontinued 2-4 days before the intervention, INR is determined once every 2 days • One day before surgery (or earlier, if the INR decreases significantly), heparin is started intravenously • For several hours before surgery, heparin is discontinued • After heparin is discontinued, low molecular weight dextran is prescribed, its infusion is continued in the postoperative period until therapy with heparin and warfarin is resumed • Therapy with heparin and indirect anticoagulants is resumed 1–2 days after surgery.
DOSAGE RECOMMENDATIONS FOR INDIRECT ANTICOAGULANTS FOR ANTITHROMBOTIC THERAPY in patients with prosthetic heart valves.
• Indications •• The first 3 months after implantation - warfarin, MHO=2.5–3.5 •• More than 3 months after implantation ••• Mechanical valves •••• Aortic implantation without risk factors: bicuspid valves (warfarin, MHO= 2.0–3.0); disc valves (warfarin, IHO=2.5–3.5); aortic implantation in combination with risk factors (warfarin, MHO=2.5–3.5) •••• Mitral implantation (warfarin, MHO=2.5–3.5) ••• Biological valves •••• Aortic implantation without risk factors (acetylsalicylic acid 80–100 mg/day) •••• Aortic implantation in combination with risk factors (warfarin, MHO=2.0–3.0) •••• Mitral implantation without risk factors (acetylsalicylic acid 80–100 mg/day) •••• Mitral implantation in combination with risk factors (warfarin, MHO=2.5–3.5) ••• Simultaneous coronary bypass surgery - addition of acetylsalicylic acid 80–100 mg/day •• • Warfarin, MHO=3.5-4.5 in high-risk patients when acetylsalicylic acid cannot be prescribed.
• If a thromboembolic episode occurs in a patient on adequate anticoagulant therapy, the dosage of drugs should be adjusted according to the following scheme •• Warfarin, MHO=2.0–3.0 - the dose of warfarin is increased until MHO=2.5–3.5 is reached •• Warfarin , MHO=2.5-3.5 - the dose can be increased until MHO=3.5-4.5 is reached.
• Proven drug interactions with warfarin •• Drugs that enhance the effect of warfarin: amiodarone, ciprofloxacin, co-trimoxazole, disulfiram, fluconazole, cimetidine, clofibrate, erythromycin, metronidazole, sulfinpyrazone •• Drugs that reduce the effect of warfarin: barbiturates, carbamazepine, griseofulvin, rifampicin , menadione sodium bisulfite.
Complications • Embolism of the arteries of the great circle • Thrombosis of the prosthesis •• In case of thrombosis of a mechanical prosthesis, the use of thrombolytics and heparin in most cases eliminates the obstruction, at least partially •• The effectiveness of thrombolysis is monitored using echocardiography •• With a high risk of bleeding and severe hemodynamic disturbances, it is preferable surgical treatment (thrombectomy or valve replacement) •• If a thrombus forms on vegetations or overgrown connective tissue, thrombolysis is ineffective or not at all effective •• Definitive recommendations for the use of thrombolysis as an alternative to surgical treatment have not yet been developed • Severe bleeding •• Occur frequently, even with carefully controlled, therapeutic level of hypocoagulation •• With long-term anticoagulant therapy, a temporary dose reduction or complete elimination of hypocoagulation is usually well tolerated by patients •• To actively eliminate hypocoagulation, menadione sodium bisulfite (5-10 mg IV), fresh frozen plasma (1-2 doses) are administered ), eliminate the cause of bleeding (using endoscopic, endovascular or surgical intervention) and resume anticoagulant therapy.
Abbreviations • IE—infective endocarditis
What may interfere with the operation?
Unfortunately, not every patient will be able to receive a donor heart. There are many different reasons for this:
- Very small number of donors. This can only happen to a person who has registered brain death and whose heart is absolutely healthy.
- There is a very long queue to receive this organ (waiting list), this is most important for children. The organ must fully comply with all the stated requirements and, perhaps, several dozen more patients who are much earlier on the list will have such parameters.
- Sometimes the donor organ cannot be delivered to the right place on time, since the operation must be performed no later than six hours after removal.
- Many people do not agree to undergo a transplant due to ethical or religious reasons. For example, in Christianity a person is alive as long as his heart beats.
- The patient can be stopped by the fear of long and rather expensive rehabilitation.
- Advanced age. Typically, operations are not performed on people over 60 years of age, but there are exceptions.
In addition to the listed obstacles, transplantation will not be performed for a number of other diseases not related to cardiology. It can be:
- pulmonary hypertension in severe form;
- diabetes mellitus at a stage in which negative changes in the retina, blood vessels or kidneys have already begun;
- infectious diseases in the acute stage;
- HIV and tuberculosis;
- autoimmune diseases - rheumatism, arthritis, lupus erythematosus, etc.;
- severe liver or kidney failure;
- chronic severe forms of lung disease;
- oncology;
- addiction to alcohol or drugs;
- severe mental disorders.
Artificial heart - contraindications for surgery
There are a number of conditions in which artificial ventricle transplantation is impossible or must be postponed:
- Infectious diseases;
- Severe renal, pulmonary, hepatic pathology;
- Disorders of the blood coagulation system, such as hemophilia.
Operating surgeons and cardiologists in Israel have extensive experience in implanting artificial ventricles. This method of correcting heart failure saves the lives of thousands of patients every year.
Please note that all form fields are required. Otherwise we will not receive your information. Alternatively use
Non-invasive ventilation
Over the past two decades, the use of non-invasive mechanical ventilation equipment has increased markedly. NIV has become a generally accepted and widespread tool for the treatment of acute and chronic respiratory failure both in hospitals and at home.
One of the leading manufacturers of medical respiratory devices is the Australian company ResMed
NIV - what is it?
Non-invasive ventilation refers to mechanical respiratory support without invasive access (ie, without an endotracheal or tracheostomy tube) using various known assisted ventilation modes.
The equipment supplies air to the patient interface through a breathing circuit. To provide NIV, various interfaces are used - nasal or oro-nasal mask, helmet, mouthpiece. Unlike the invasive method, the person continues to breathe on his own, but receives hardware support during inspiration.
When is non-invasive ventilation used?
The key to successful use of noninvasive ventilation is recognition of its capabilities and limitations, as well as careful patient selection (diagnosis and patient assessment). Indications for NIV are the following criteria:
- shortness of breath at rest;
- respiratory rate RR>25, participation of auxiliary respiratory muscles in the respiratory process;
- hypercapnia (PaC02>45 and its rapid increase);
- Ph level
- symptomatic lack of positive effect from oxygen therapy, hypoxemia and gas exchange disorders;
- increase in airway resistance by 1.5-2 times the norm.
To perform non-invasive ventilation, the patient must be conscious and able to follow the instructions of the doctors. There must be a clear prospect of stabilizing the patient within several hours or days after the start of respiratory support. Absolute contraindications for NIV are:
- coma;
- heart failure;
- respiratory arrest;
- any condition requiring immediate intubation.
Advantages of NIV
One of the advantages of non-invasive ventilation is the ability to carry out therapy at home.
Non-invasive ventilation allows you to help a patient with acute or chronic respiratory failure without resorting to endotracheal intubation or tracheostomy. The technique is simpler and more comfortable for the patient. Let us list the main advantages of NIV.
- A respiratory support session is easy to start and just as easy to complete.
- The patient retains the ability to speak, swallow, eat independently, and cough.
- The procedure does not cause complications that are possible with endotracheal intubation and tracheostomy, including mechanical damage to internal organs by the tube, bleeding, swelling of the glottis, infection of the respiratory tract, etc.
- The air passes through the respiratory tract, due to which it is humidified, purified and warmed naturally.
- NIV can be performed at an early stage of the disease, i.e. before the patient's condition becomes critical. This shortens the duration of treatment, reduces the number of complications, and also reduces the risk of readmission.
- In many cases, devices for non-invasive respiratory support can be used not only in a hospital, but also at home.
- There is no “respirator weaning” period after completion of treatment.
Non-invasive conscious ventilation also has some disadvantages and side effects. For example, it is impossible to apply high treatment pressure, because this leads to significant leakage from under the mask. There is no direct access to the respiratory tract, so it is impossible to sanitize it. It is also impossible not to mention the likelihood of aerophagia, aspiration of stomach contents and skin irritation in the areas where the contour is adjacent.
Non-invasive ventilation in CPAP and BIPAP modes
The terms CPAP and BIPAP are often used interchangeably with NIV. These are common methods of non-invasive respiratory support using special portable devices. Many modern ventilators used in intensive care units have the option of CPAP and BIPAP.
Portable respirators are low cost (relative to resuscitation stationary ventilators), and they effectively compensate for even high air leakage. But most often they do not provide advanced monitoring of the patient's condition in real time.
Most resuscitation respirators can operate in CPAP and BIPAP modes. But more often, portable devices are used to provide respiratory support to a conscious patient.
In CPAP (continuous positive airway pressure) mode, the device supplies air under constant positive pressure, and the patient breathes spontaneously (i.e., independently). The method is used in the management of patients with moderate to severe obstructive sleep apnea syndrome (OSA), as well as post-traumatic or postoperative acute respiratory failure.
Bi-level positive airway pressure (BIPAP) devices have a wider range of applications and different mode options. Unlike CPAP, they involve an increase in pressure as you inhale and a decrease in pressure as you exhale. Thanks to this, it becomes possible to use high treatment pressure, but the patient does not experience discomfort in the exhalation phase, overcoming the resistance of the air flow. Two-level ventilation allows you to relieve the respiratory muscles, reduce the respiratory rate and increase the tidal volume. And the presence of auxiliary modes in modern models helps to select the optimal treatment protocol in accordance with the diagnosis and needs of the patient.
CPAP and BIPAP machines to help patients with COVID-19
Over the past few months, the issue of mechanical ventilation has been raised frequently in connection with the COVID-19 pandemic. High demand for ventilators has caused their shortage. The Australian company ResMed, as a manufacturer of medical respirators, is taking the necessary measures to prioritize the production of devices to assist patients with severe respiratory failure. But due to the acute shortage of equipment at the moment, alternative ventilation options, incl. non-invasive respiratory support.
The COVID-19 pandemic has led to a shortage of ventilators. In this regard, primary care for patients with coronavirus infection and symptoms of acute respiratory failure can be carried out using CPAP and BIPAP machines.
CPAP and bipap therapy can be used to provide primary care to patients with COVID-19 who require respiratory support. According to clinical protocols and reports received from clinicians in Italy and China, non-invasive ventilation (including BPAP and CPAP) for patients with COVID-19 is recommended in the following scenarios.
- To provide respiratory support to patients with respiratory failure who have not yet progressed to more severe hypoxemia.
- To facilitate extubation and recovery after invasive ventilation.
- To reduce hospital stays by allowing patients who still require respiratory support and rehabilitation to transition to home care.
NIV cannot replace invasive ventilation in the most severe forms of COVID-19. But this therapy is important when triaging patients in medical institutions. CPAP and BIPAP machines provide supplemental oxygen for less severe cases and reduce dependence on invasive ventilation. In addition, they are relevant for countries where hospital bed capacity turned out to be insufficient during the ongoing pandemic.
The following ResMed brand devices are suitable for home therapy: Lumis series BIPAP devices, as well as the AirCurve 10 CS PaceWave servo ventilator. They can be connected to additional oxygen (up to 15 l/min), as well as a module with a pulse oximetry sensor to monitor blood oxygen saturation.
Long-term use of non-invasive ventilation: benefit or harm
There is an opinion that the longer a patient is on a ventilator, the more difficult it is for him to refuse a respirator. This gives rise to the fear of “forgetting how” to breathe without a device and the fear of suffocation if the device turns off for some reason. But such risks occur only with invasive ventilation, when the device literally breathes instead of the patient. In turn, long-term use of non-invasive ventilation does not cause addiction, because the patient breathes on his own, and medical equipment only helps him with this.
According to research results, long-term non-invasive respiratory support (including at home) can optimize gas exchange, reduce the load on the respiratory system and reduce the risk of subsequent hospitalizations in patients with COPD. One of the advantages of long-term NIV is the ability to provide rest to the respiratory muscles, which are in a state of chronic fatigue.
Long-term use of non-invasive ventilation improves sleep quality and well-being while awake. When NIV is discontinued even for a week, patients with chronic respiratory failure begin to have morning migraines again, shortness of breath appears, and their night saturation also worsens.
NIV for chronic respiratory failure is most often performed at night. First, it increases the total time of respiratory support. Secondly, it helps eliminate nocturnal hypoventilation and episodes of desaturation, which most often occur in the REM phase of sleep.
Preparing the patient for surgery
Heart transplantation is a complex procedure that requires careful preparation. The patient undergoes a multi-stage examination before a decision is made about the possibility of organ transplantation. Necessary:
- carefully collect anamnesis of the disease;
- perform a chest x-ray;
- take a maximal oxygen uptake (VO2) stress test;
- undergo a series of tests for infections to exclude hepatitis, HIV and a number of other diseases that may be a contraindication;
- undergo routine urine and blood tests to assess the general condition of the body;
- undergo cardiac catheterization with tonometry of the right side (necessary to exclude pulmonary hypertension, which will be a contraindication to transplantation);
- take a test to evaluate human lymphocyte antigen (HLA);
- undergo an ECG, EchoCG.
The examination may reveal both absolute and relative contraindications that exclude the patient from being placed on the waiting list for a donor heart.
4.Risks of heart transplant
The main risks of heart transplant surgery include:
Rejection of a donor heart.
To check the patient's body, surgeons regularly perform biopsies of heart tissue, as well as echocardiography, electrocardiography, or blood tests.
If the patient's body rejects the heart, additional medications (immunosuppressants or steroids) are prescribed to suppress the immune system so that it will accept the donor heart. These medications may have side effects, the most serious of which are various infections and the development of cancer.
Atherosclerosis of the arteries, which could appear in the donor heart
. This is usually a complication and at the same time an important limiting factor that affects life expectancy.
Things to think about
After a heart transplant, you must follow strict life rules, which include taking daily medications and regular medical care. Medical care involves ongoing testing (biopsies) of tissue from the transplanted heart to prevent rejection.
Transplant candidates receive a heart based on the date of registration and the severity of the disease. Also, do not forget that the number of donor organs is limited.