CA-19-9 is a protein produced by pancreatic tumor cells. Normally, it is present in the tissues of various organs, primarily related to the gastrointestinal tract. The glycoprotein is also found in seminal fluid. Deciphering the result for the cancer antigen CA-19-9 must be approached very carefully, since an increase in its level cannot be interpreted unambiguously.
For example, in women after 50 years of age, the protein level may be higher than normal, and in some representatives of the Caucasian peoples, due to a genetic feature, the tumor marker in the blood serum is completely absent, since it is not produced by the body. The analysis is not suitable for accurate differentiation of the disease and early diagnosis of tumors, so it is carried out in combination with the study of other antigens - CA-125, HE4, AFP, CEA.
Specifics of the study
The first thing you need to know about this test is that its results are not definitive proof of cancer development.
This antigen does not belong to the category of specific and sensitive exclusively to mutated cells. Protein specificity rates range from 75 to 100 percent, which, combined with sensitivity of 68 to 93 percent, makes false-positive rates likely. However, if the norm is exceeded a hundred times or more, there is a 96 percent chance of having cancer. Once the problem is detected, the doctor can prescribe adequate therapy. Scientific studies show that those patients whose test results showed a decrease or stabilization of indicators have a better prognosis than patients with increasing values.
Among the distinctive features of the study it is worth noting:
- the need for additional screening diagnostics;
- the likelihood of undetecting a pathogenic protein in a person with a certain blood type, even in the presence of tumors and metastases;
- often carried out in combination with CEA or CA 72-4, if the doctor thinks that the patient has a neoplasm in the intestines or gastric carcinoma.
So, you now know what antigen means, so you can begin to determine which categories of patients are prescribed for examination.
Cancer antigen 125 (CA 125)
Indications for the use of cancer antigen CA 125:
- Monitoring the course and effectiveness of therapy for serous ovarian carcinoma
Cancer antigen CA 125 is a glycoprotein with a molecular weight of 200,000 Da and is a differentiation antigen that originates from derivatives of the coelomic epithelium of fetal tissues. The upper limit of normal for the cancer antigen CA 125 is 35 U/ml, but where increased specificity is required, this limit rises to 65 U/ml.
Increased levels of cancer antigen CA 125 are observed in the blood serum of patients with ovarian cancer. However, a significant increase is also observed in tumors of the gastrointestinal tract, bronchial carcinoma, and breast carcinoma. Sometimes the level of cancer antigen CA 125 is increased during inflammatory processes involving the appendages, and with benign gynecological tumors. A slight increase in this marker is noted during pregnancy, with autoimmune diseases, hepatitis, chronic pancreatitis and cirrhosis of the liver.
Purpose of the procedure
The most common cases when there is a need to check compliance with the antigen standard:
- Prevention of cancer pathologies in people with an unfavorable family history - if there have been cases of gastric carcinoma and similar diseases in the family, the health status must be closely monitored.
- Obvious symptoms of the development of a neoplasm in the pancreas or already identified cancer.
- Detection of recurrent disease.
- Monitoring the degree of effectiveness of anti-cancer therapy - based on the decoding of the analysis and the difference from the norm of the tumor marker Ca 19-9, the doctor can understand whether the prescribed treatment has the desired effect, and, if necessary, make adjustments to the regimen.
- Checking the assumption of the development of malignant or benign neoplasms in the liver, gall bladder, gastrointestinal tract, and bile ducts.
- Determining the dynamics of the patient's condition.
- Detection of metastases before characteristic signs of disease spread appear.
In addition to cancer, the study can detect cystic fibrosis, cholecystitis in the chronic stage, cirrhosis of the liver and gallstones, as well as a number of inflammatory pathologies. An experienced oncologist knows exactly what the Ca 19-9 tumor marker shows and can use the data obtained to prescribe other necessary examinations and establish a diagnosis. Often, it is the decoding of this analysis that helps to identify the disease at a stage when a clear clinical picture has not yet formed. It is much easier to defeat cancer at an early stage, so if the doctor prescribed it, it means there is a need for it.
CA 19-9 is a specific antigen produced by epithelial cells of the gastrointestinal tract.
Synonyms Russian
Carbohydrate antigen 19-9, cancer antigen CA 19-9.
English synonyms
Ca 19-9, Cancer Antigen 19-9, Carbohydrate Antigen 19-9, Gastrointestinal Cancer Antigen.
Research method
Chemiluminescent immunoassay.
Determination range: 0.6 - 10000 U/ml.
Units
U/ml (unit per milliliter).
What biomaterial can be used for research??
Venous blood.
How to properly prepare for research?
Do not smoke for 30 minutes before the test.
General information about the study
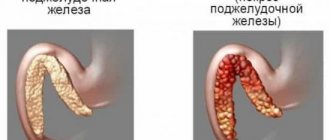
Cancer antigen CA 19-9 is a high molecular weight glycoprotein that is normally produced by epithelial cells of the gastrointestinal tract. Its level increases in almost all patients with tumors of the gastrointestinal tract, especially the pancreas. Produced by tumor cells, CA 19-9 enters the bloodstream, making it an effective tumor marker for monitoring the course of the disease.
The level of the tumor marker CA 19-9 is elevated in 70% of patients with pancreatic cancer.
Its concentration may also increase in tumors of other locations (colorectal cancer, cancer of the liver, stomach, gall bladder or bile ducts, ovaries), liver diseases (hepatitis, cirrhosis), cholelithiasis, pancreatitis, cystic fibrosis.
The CA 19-9 test is not used for the primary diagnosis of cancer, as it does not have sufficient sensitivity and specificity.
What is the research used for?
- To monitor the effectiveness of treatment for pancreatic cancer and detect relapses of the disease.
- To obtain information about the prevalence of the tumor process, the presence of distant metastases in pancreatic cancer.
- For the differential diagnosis of pancreatic cancer with other diseases such as pancreatitis.
When is the study scheduled?
- For symptoms of pancreatic cancer: abdominal pain, nausea, weight loss, jaundice.
- Periodically to monitor the effectiveness of treatment and detect relapses in patients with pancreatic cancer with initially elevated CA 19-9 levels.
- If you suspect cancer of the liver, gall bladder or bile ducts, stomach, colon (in combination with other tumor markers).
What do the results mean?
The isolated use of research for the purposes of screening and diagnosing cancer is unacceptable. The information in this section cannot be used for self-diagnosis and self-treatment. Diagnosis of any disease is based on a comprehensive examination using various methods, not just laboratory ones, and is carried out exclusively by a doctor.
Reference values: 0 - 34 U/ml.
The absence or low level of cancer antigen CA 19-9 in the blood is typical for healthy people.
Excessive levels of the tumor marker CA 19-9 in the blood most often indicate pancreatic cancer. Typically, the higher a patient's CA 19-9 level, the more advanced the disease they are. A very high concentration of CA 19-9 is observed in the case of metastatic pancreatic cancer.
In addition, a high CA 19-9 level may indicate various diseases: colorectal cancer, cancer of the liver, stomach, gall bladder or biliary tract, ovaries, liver disease (hepatitis, cirrhosis), cholelithiasis, pancreatitis, cystic fibrosis.
However, a normal CA 19-9 concentration does not exclude the presence of pancreatic cancer. This happens at the initial stage of the tumor process, when the level of CA 19-9 has not yet increased.
Periodic measurements of CA 19-9 may be useful during and after treatment for pancreatic cancer. Based on the increase or decrease in its level, the effectiveness of the treatment can be assessed or tumor relapses can be detected.
The absence or reduced content of cancer antigens CA 19-9 in the blood means:
- the norm
- the success of the treatment,
- early stage of pancreatic cancer, when the level of tumor markers has not had time to increase.
An increased level of cancer antigens CA 19-9 in the blood means:
- pancreas cancer,
- tumor of other localizations (colorectal cancer, cancer of the liver, stomach, gallbladder or biliary tract, ovaries),
- liver diseases (hepatitis, cirrhosis),
- cholelithiasis,
- pancreatitis,
- cystic fibrosis.
What indicators are considered normal?
The international system of standards has not established the norm of the tumor marker Ca 19-9 in the blood. Differences in acceptable values are associated with variability in the methods for studying the biomaterial and the reagents used. If you carefully study the survey form, you will see that the standard values are indicated in the reference indicators field. For women and the stronger sex, the norm is identical and averages 10-37 (sometimes up to 35) units per milliliter.
Attention! Given the narrow limits of the norm, the analysis with decoding should be carried out for the first and subsequent times in the same medical center.
Excess in various pathologies
The likelihood of readings being higher than normal varies depending on the disease.
Mostly upward jumps in values occur when foci of cell mutation form in organs such as the gallbladder (in 90% of cases), mammary gland (70%), liver (90%), sigmoid and rectum (90%), pancreas (100%). %), ovary (70%), bile ducts, uterus. In such patients, during decoding, an increase in the tumor marker Ca 19-9 to 500 units/ml and above is recorded. As for benign processes, their values do not exceed the specified limit, and the chance that exceeded parameters indicate a disease is 50 to 50. Among the diseases during which the antigen in the blood becomes more than normal, there are acute, chronic and toxic forms hepatitis, cirrhosis, hereditary damage to the exocrine glands, cholelithiasis, gastrointestinal tract infections and cholecystitis.
Tumor marker CA 19-9
Early diagnosis of cancer can significantly increase the chances of not only five-year survival, but even a complete cure. Among all cancers of the colon, it is especially common. Pancreatic cancer is also a common cancer. One of the methods for detecting these pathologies in the early stages is the analysis of the CA 19-9 tumor marker.
Tumor markers - what are they?
Tumor markers are protein compounds that are produced by malignant cells and surrounding tissues. Hormones, enzymes, waste products, particles of cell membranes and other structures play the role of antigens. Using them, the immune system detects pathology and eliminates it in a timely manner.
If the malignant process turns out to be too aggressive, the immune system can no longer cope with it. The level of antigens is growing rapidly. It is this growth that is an important diagnostic factor in oncology.
By examining the tumor marker CA 19-9 and other antigens, it is possible to detect cancer in the early stages, which will significantly increase survival. They also help determine the malignancy of the process and make prognoses for the patient. Testing for tumor markers plays an important role in monitoring the effectiveness of already prescribed treatment and timely detection of metastases or relapses.
Reliability of results
Protein CA 19-9 is the main tumor marker for damage to the pancreas, liver, bile ducts, sigmoid and rectum. It is worth remembering that the reliability of the test is only 70-80%. If cancer of the digestive organs is suspected, an analysis of this substance is prescribed along with CEA and AFP (other intestinal tumor markers). In 10% of patients with cancer of the pancreas, liver, and colon, CA 19-9 is not produced at all, even in the last stages of the disease.
The increase in the level of this protein is affected by many other, even non-malignant, diseases:
• Pancreatitis; • Hepatitis and cirrhosis of the liver; • Cholelithiasis; • Colitis and enteritis of various etiologies.
If, according to the results of the analysis, CA 19-9 is elevated, this is not yet a reason to diagnose yourself with cancer, but the need to undergo a more detailed examination, including ultrasound, elastography, MRI and biopsy followed by histology.
Indications for taking the test
Analysis for the tumor marker CA 19-9 is prescribed for certain indications.
They are determined by the doctor based on the patient’s complaints, symptoms and other diagnostic tests: • Symptoms of pancreatic disease – acute pain in the left hypochondrium, persistent nausea and vomiting; • Symptoms of liver disease – jaundice, pain in the right hypochondrium, heartburn; • Intestinal disorders, diarrhea or constipation, pain in the lower abdomen; • Increased blood tests for amylase, AST, ALT, alkaline phosphatase and others.
In addition to suspicions of digestive cancer, the test is regularly prescribed to people over 50 years of age who have a family history of relatives with cancer, patients undergoing treatment, and survivors of surgery.
Do I need to prepare to donate blood?
To obtain a more accurate and reliable result, you need to prepare for donating blood.
This is not difficult to do: • A week before taking blood, you must avoid drinking alcohol; • Limit physical activity for several days, do not overheat, do not visit saunas and solariums; • At least 3-4 days in advance, give up fatty, spicy, salty, smoked foods; • One day before donating blood, you must stop taking any medications (in consultation with your doctor) or, if this is impossible for health reasons, inform about this before the test; • The last meal should be 12 hours before taking the material. You can drink regular, still water on the day of the test; • You should not smoke before donating blood.
Decoding the results of CA 19-9
Only a doctor should evaluate the test results. It is based not on bare numbers, but also on technology, methodology, patient history, examination, and data from other studies. Laboratories may have different equipment installed and different reagents used, which is why the norms may also differ. Therefore, it is better to take repeated tests at the same medical institution.
On average, it is believed that the level of CA 19-9 is less than 34-37 U/ml - the norm (in some laboratories up to 39 U/ml). Deviations can fluctuate within 500 U/ml, which is not yet an indicator of cancer, but requires a detailed examination of the patient. An increase in the tumor marker level above 1000 U/ml can already indicate oncology of the second stage and higher, with the formation of metastases.
Monitoring fluctuations in these numbers allows you to evaluate the effectiveness of treatment, timely monitor the onset of relapse, and make a prognosis for the patient’s survival.
Where to donate blood for the CA 19-9 tumor marker?
Tests for the CA 19-9 tumor marker can be done in several medical institutions:
• Municipal clinics. Benefits - free. Disadvantages - you need a doctor’s referral, queues can be months in advance, unfriendly staff, tired from the excessive workload, low reliability of the results; • Private laboratories. Advantages: accurate results, polite employees, modern equipment. Disadvantages - you will have to look for a doctor to interpret the tests and consult on your own; • Private specialized clinics. Advantages - reliable results, high-precision equipment, high-quality reagents, no queues, polite, qualified staff, the opportunity to immediately, on the spot, undergo consultation and additional examination. Disadvantages - none.
In our clinic you can undergo a full diagnosis, receive consultation and treatment, as well as professional psychological support, which is especially important for patients with suspected cancer.
Recommendations for preparation
The informativeness and reliability of the data during the examination equally depends on the quality of the equipment, the professionalism of the laboratory technicians and the behavior of the patient himself. To avoid receiving false positive results, you must:
- inform the doctor about the need to take any medications - if possible, then you should stop taking medications for a day. If the drugs cannot be stopped, the doctor will take this into account when interpreting the analysis for Ca 19-9 tumor markers;
- do not drink alcoholic beverages for three days before the procedure, and do not smoke on the day of blood sampling;
- review your diet - so that the indicators are informative, it is undesirable to eat spicy and fried foods, give up exotic foods and fatty foods;
- come to the laboratory hungry - biomaterial is taken on an empty stomach, so the last meal should be 8 hours before;
- calm down - nervous experiences affect the composition of the blood, so before entering the manipulation room, sit for a couple of minutes in the hall;
- do not engage in heavy physical labor for about three days (this also includes intense sports training);
- warn if within 7 days before the procedure you underwent ultrasound diagnostics, magnetic resonance or computed tomography.
How are the results deciphered?
It is up to the specialist to determine what the tumor marker Ca 19-9 shows. On medical forums you can find a large amount of information about what diseases provoke deviations from the norm, but without the help of a specialist it will not be possible to decipher them, as well as to select effective treatment tactics. Therefore, even knowing what an increase in indicators means, the patient should trust the doctor, who will carefully study the laboratory results and give an expert opinion taking into account a number of factors:
- age and gender of the patient;
- state of the body at the time of examination;
- presence of other diseases;
- data from other instrumental and biochemical studies;
- characteristics of the equipment and reagents used;
- previously detected cancer;
- family history;
- changes in normal values due to the need for the patient to take any medications.
No doctor will simply prescribe such a test, so if you receive a referral, you must immediately contact the laboratory. Israeli clinics are considered the best in early diagnosis of oncology and delight clients with reasonable prices. By contacting a reliable medical center like Assuta, you can count on prompt and highly accurate diagnostics, as well as attractive conditions for further treatment.