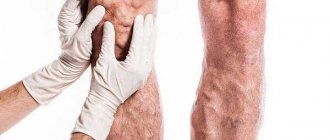
Pain in the legs due to varicose veins is a complaint that patients often present when visiting doctors. In the presence of varicose leg pain, phlebologists at the Yusupov Hospital conduct a comprehensive examination of patients using physical and instrumental diagnostic methods. For ultrasound examinations, the latest devices from the world's leading manufacturers are used, which have high resolution and allow the patient to be examined in one, two or three modes.
Conservative treatment of patients who have pain in varicose veins in the legs is carried out with the latest generation of medicines. Their effectiveness and safety have been proven by scientific research. Highly qualified surgeons and the presence of modern equipment and surgical instruments in the phlebology department allow phlebologists to perform operations using both classical and innovative techniques.
Severe cases of varicose veins, when pain due to varicose veins in the legs is not relieved by medications, are discussed at a meeting of the expert council with the participation of candidates and doctors of medical sciences, doctors of the highest qualification category. Leading specialists in the field of diseases of the peripheral venous system collectively decide how to reduce pain from varicose veins of the legs. If pain in a vein in the leg due to varicose veins does not go away as a result of adequate conservative therapy, patients are offered surgical intervention. Surgeons perform the operation only after obtaining the patient's informed consent.
Causes
Varicose veins of the lower extremities are a disease that develops for various reasons. The following factors play a role in the formation of pathological changes in the inner lining of the venous vessel:
- burdened heredity;
- obesity;
- hormonal imbalances;
- pregnancy;
- wrong lifestyle;
- chronic constipation.
- uncontrolled use of hormonal contraceptives;
- systematic sports activities.
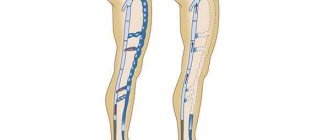
The main reason for the development of varicose veins is changes in the wall and valves of the veins.
With valvular insufficiency of various parts of the venous bed of the lower extremities, a pathological, retrograde (in the opposite direction) blood flow appears. It is the main factor in damage to the microvasculature. In the presence of various risk factors and under the influence of gravity, the pressure in the venous knee of the capillary increases, which reduces the arteriovenular gradient necessary for normal perfusion of the microvasculature (passing blood through the vascular wall). The consequence is first periodic and then constant oxygen starvation of tissues. Patients with varicose veins have severe pain in their legs because the soft tissues do not receive oxygen, swelling occurs, which compresses the nerve fibers.
In the future, with varicose veins, trophic disorders develop. Varicose veins are accompanied by severe pain in the legs at night. With the development of a trophic ulcer, the pain intensifies. Patients are forced to sleep with a pillow under their feet. If inflammation of the soft tissues around the trophic ulcer occurs, the pain becomes unbearable. In some cases, doctors are forced to prescribe opiates to relieve pain.
Make an appointment
Causes and prevention of varicose veins of the lower extremities
The causes of varicose veins in most cases lie in a sedentary lifestyle: we spend a long time in a static position (especially sitting), and do not give the necessary stress to our muscles. The following risk factors may also affect:
- excessively tight clothing (pants, socks),
- uncomfortable shoes (especially high heels),
- disturbances in hormonal balance,
- frequent hot baths, sauna visits,
- prolonged serious physical activity, heavy lifting,
- obesity.
The main prevention of varicose veins is maintaining a healthy lifestyle. Even if you have a sedentary job, try not to sit without moving for several hours in a row: get up, stretch your legs, walk more; and don’t forget about a balanced diet, which, along with moderate physical activity, will protect you from many diseases.
Pregnancy is also one of the serious risk factors for varicose veins. But genetic predisposition is still being studied, and there are even a number of studies that refute it.
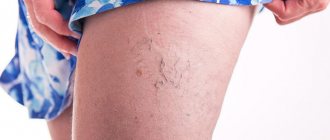
Symptoms
Chronic venous insufficiency is manifested by the following main symptoms:
- pain in the calf muscles of the legs due to decreased tone of the venous wall and oxygen starvation;
- heaviness in the lower extremities;
- convulsions;
- swelling of the legs in the evening due to overload of the lymphatic system, increased capillary permeability and inflammatory reactions;
- constant skin itching.
A characteristic feature of pain in chronic venous diseases is its localization in the calf muscles. Venous pain in the legs is never sharp and does not radiate to the thigh or foot. The pain is moderate, nagging, dull. They appear in the evening and disappear after a night's rest. Acute pain in the lower extremities in patients with varicose veins occurs due to inflammation of the vein (thrombophlebitis) or thrombosis of the deep veins of the leg.
Cure for varicose veins
The vast majority of people, choosing between surgical treatment of any disease (in our case, varicose veins) and possible non-invasive treatment, be it capsules, tablets for varicose veins, gels, ointments, herbs and infusions, will choose the second, especially if from all screens will tell them about their effectiveness and the unnecessaryness of any operations. The amount of information used to intoxicate patients can be judged by the amount of advertising of tablets and creams for varicose veins on all federal channels, where happy girls easily run after lubricating their healthy legs with a miracle cream or taking miracle pills.
Diagnostics
When examining patients who have leg pain in the vein area, phlebologists pay attention to the location of the pain and the color of the skin over the vein.
If a vein appears on the leg and it hurts, an ultrasound scan is performed. Doctors use 1, 2 or 3 research modes. The main one is B-mode. Additionally, color coding of blood flow or pulsed wave Doppler, as well as a combination of both, is used. The method allows you to simultaneously examine the vein in the leg that hurts, determine the direction of blood flow and its parameters. Patients who have pain and stretching of the veins in their legs must undergo an examination of the superficial and deep veins of both lower extremities. At the Yusupov Hospital, doctors for ultrasound scanning use devices equipped with linear sensors with a frequency of 5 MHz and more from the world's leading manufacturers. To scan deep veins, especially in obese patients, convex sensors with a lower radiation frequency (from 3.5 to 5.0 MHz) are used.
Doppler ultrasound allows you to obtain sound information, which doctors use to judge the presence or absence of blood flow through the main veins. By changing the sound signals during functional tests, blood reflux (reverse discharge) is detected. During X-ray contrast venography, the phlebologist examines the deep and superficial veins. He receives comprehensive information about the morphological changes of the venous system. This research method is used when planning surgery in patients with blocked or underdeveloped veins. Phlebologists at the Yusupov Hospital use the technique of transfemoral ascending phlebography.
Radionuclide phlebography allows you to obtain data on the nature and direction of blood flow through deep, superficial and perforating veins under conditions that are as close as possible to physiological ones. The study is carried out with the patient standing while simulating walking. Radiophlebography allows for an integrative assessment of blood flow throughout the entire system simultaneously.
Intravascular ultrasound examination is carried out to determine the shape and extent of the narrowed segment of the vein when its patency is impaired after thrombosis or compression of the venous vessel from the outside. Infrared thermography, computer thermal imaging, radiothermometry are used as an additional method of studying patients whose cause of pain in the veins of the legs is chronic venous insufficiency. Using this method, the dynamics of the inflammatory process in tissues is monitored, as well as the effectiveness of therapeutic measures is assessed.
Venous pain - occurrence and treatment
An international group of experts with the participation of the American Venous Forum (AFR), the European Venous Forum (EVF), the International Union of Phlebologists (UIP), and the International Union of Angiologists (IUA) presented a unified terminology characterizing the various manifestations of CVD, recommended for treatment and research work. Thus, it is proposed to distinguish between soreness or pain, a burning sensation, muscle cramps, a feeling of swelling or pulsation, a feeling of heaviness, itching, restless leg syndrome and fatigue [1]. Since all these complaints are not specific, their connection with chronic venous disease is highly likely under the following conditions: direct dependence on the nature of daily activity and the duration of static loads, ambient temperature and hormonal fluctuations in women.
An important sign is the reduction or disappearance of these symptoms after resting or raising the lower extremities above the horizontal level [2]. In addition, a thorough clinical and instrumental examination of the venous system of the lower extremities is necessary.
Specific symptoms of CVD are rarely the subject of epidemiological studies and, as a rule, they are assessed in aggregate. According to various data, the frequency of subjective complaints associated with chronic venous disease occurs in 29-61% of respondents, clearly prevailing in women.
Pain and venous pain: definition and mechanism of development.
As for individual symptoms, in particular venous pain, the range of data is even wider. Thus, Ruckley et al. [3] reported 44% of cases identified by review of medical records. Jawien et al. [4] during a non-specialized examination found venous pain in 75.7% of patients. Langer et al. [5] in a survey of employees at the University of California found pain associated with CVD in 18%. It should be emphasized that pain is often the main reason for patients to see a doctor.
What is venous pain and what is the mechanism of its development? The International Association for the Study of Pain (IASP) defines pain as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage or described in terms of such damage.” In other words, pain is usually more than a sensation associated with existing or possible organic damage, since it is accompanied by an emotional component.
There are acute and chronic pain.
- Acute pain is defined as “pain of short duration of onset with an easily identifiable cause.” Acute pain is a warning to the body about the current danger of organic damage or disease.
- Chronic pain was originally defined as “pain that lasts about 6 months or more.” Now, it is pain that “persistently persists beyond the appropriate length of time during which it should normally end.” Previously, it was believed that pain is not a specific physical sensation, and therefore there are no special receptors that perceive only painful stimulation.
That is, the appearance of a feeling of pain can be caused by irritation of any type of nerve endings, if the force of irritation is strong enough. The existence of specialized bolinocyceptor receptors, characterized by a high perception threshold, has now been proven. They are excited only by stimuli of “damaging” intensity.
All nociceptors are free nerve endings that consist only of the terminal branches of the axial cylinder of the sensory neuron, which is why they got their name, and they are located outside the spinal cord stem, but in the dorsal ganglion. The concentration of nociceptors varies throughout the body. They are mainly distributed over the surface of the skin, in ligaments or joint capsules, and are least often found in deeper tissues and organs. Nociceptors recognize mechanical, thermal and chemical stimuli without adapting to them. That is, different individual pain sensitivity thresholds are associated with the emotional and subjective characteristics of the human psyche.
Nociceptive nerves contain small-diameter primary fibers that have sensory endings in various organs and tissues. There are two main classes of nociceptors, Aδ- and C-fibers, which transmit fast and slow pain sensations, respectively. The class of Aδ-myelinated fibers (covered with a thin myelin coating) conduct signals over a distance of 5 to 30 meters per second, serving to transmit rapid pain. This type of pain is felt within one tenth of a second from the moment the painful stimulus occurs. Activation of Aδ-nociceptors occurs as a result of strong mechanical or thermal effects. Slow pain, transmitted through slower, unmyelinated (“naked”) C-fibers that send signals 0.5 to 2 meters per second, is an aching, throbbing, burning pain—such as venous pain. C-nociceptors perceive mechanical, thermal and chemical stimulation.
There are also so-called “sleeping” nociceptors, which are activated during inflammation. Nociceptors have a certain threshold of sensitivity, that is, a certain minimum level of stimuli is required before they lead to the generation of a signal. In some cases, the excitability of pain fibers becomes excessive, exceeding the actual level of exposure to the painful stimulus, which leads to a condition called hypersensitivity to pain - hyperalgesia. Once the threshold is reached, the signal is transmitted along the nerve axon to the dorsal horn of the spinal cord, where the primary processing of sensory information occurs. Then, nociceptive impulses are transmitted through interneurons to the cells of the anterior and lateral horns, causing reflex motor and autonomic reactions. Another part of the impulses excites neurons, the axons of which form ascending pathways. At the level of the hypothalamus and limbic complex, the formation of emotional and behavioral reactions, autonomic and endocrine shifts that accompany pain occurs. The final analysis of incoming nociceptive information is carried out by the cortex of the parietal, frontal and temporal lobes of the brain.
Along with the nociceptive system, there is its antagonist, the antinociceptive system, which suppresses pain impulses. It includes the structures of the cerebral cortex, the diencephalic level, the periventricular and periaqueductal gray matter (rich in enkephalin and opiate neurons), some nuclei of the reticular formation of the brain stem, in which serotonin and norepinephrine act as neurotransmitters. The participation of serotonin and norepinephrine in the functioning of the antinociceptive system explains the reduction of pain caused by a number of drugs (tricyclic antidepressants, etc.) that suppress reuptake at serotonergic and norepinephrine synapses and thereby enhance the descending inhibitory effect on neurons of the dorsal horn of the brain.
Thus, under normal conditions, there is a harmonious relationship between the intensity of the stimulus and the response to it at all levels of organization of the pain system. An imbalance between the nociceptive and antinociceptive systems forms the sensation of pain either due to the activation of the first, or due to the insufficiency of the second, which gives rise to its pathophysiological changes. In terms of temporal characteristics, this relationship can manifest itself in both acute and chronic pain.
The point, however, is not so much in temporal differences as in qualitatively different neurophysiological, psychological and clinical features of the pain syndrome. Thus, acute pain is always a symptom, but chronic pain can essentially become an independent disease. Chronic pain in its pathophysiological basis may have a pathological process in the somatic sphere, primary or secondary dysfunction of the peripheral system, or the central central nervous system, it can also be caused by psychological factors. According to the IASP, pain is a more general concept that includes both objective (associated with nociception) and subjective experiences that are usually accompanied by nociception, but can also occur without any stimuli.
As noted above, nociception (synonyms: nocireception, physiological pain) is activity in the afferent nerve fibers of the peripheral and central nervous system, excited by a variety of stimuli with “damaging” intensity. Nociception is a neurophysiological concept that refers to the perception, conduction and central processing of signals about harmful processes or influences. That is, this is a physiological mechanism for transmitting pain, and it does not affect the description of its emotional component. It is important that the transmission of pain signals in the nociceptive system itself is not equivalent to perceived pain. In other words, patients with CVD can perceive a nociceptive signal in some cases as pain, and in others as burning, pulsation, a feeling of heaviness and other difficult-to-describe sensations.
Pathogenesis of venous pain.
For a long time there was an opinion that peripheral veins do not have pain receptors. Only relatively recently, using electron microscopy, sensory fibers were discovered in the wall of peripheral veins, the cell body of which is located in the dorsal roots of the spinal cord [6]. Sensory fibers pass along the venous wall and are divided into numerous collaterals. Some of them penetrate the adventitia and end in the venous wall between the intima and media. Others penetrate paravasal tissues and end in demyelinated nerve endings that are in close contact with the vessels of the microvasculature. Subendothelial and paravasal nerve endings are nociceptors that transmit afferent signals generated in the venous wall and paravasal tissues.
That is, the pain syndrome that occurs as a result of pathological processes in the venous wall is associated with irritation of nociceptors. Various agents can act as a trigger. In particular, clinical experiments demonstrate that venous pain can be caused by mechanical stimuli such as venipuncture and catheterization, as well as chemical irritation resulting from the administration of cold (<20°C), hyperosmolar, acidic (pH<4) or alkaline solutions (pH> 11) [7].
In addition, inflammation of the venous wall (phlebitis and thrombophlebitis), as well as thrombus formation, can be an inducer of pain syndrome. In some cases, hypoxia stimulates the synthesis of various proinflammatory mediators, which in turn activate subendothelial and paravasal nociceptors. Such substances are called algogens. Venous pain can be studied in vivo. Thus, in a healthy person, a section of the saphenous vein of the forearm is isolated by applying pneumatic cuffs that compress the adductor and efferent segments. Additionally, local anesthesia is performed to block sensory nerve fibers.
Using this model, it was shown that pain occurs when the diameter of the vein is mechanically increased (using a balloon) by more than 3 times. This phenomenon is also clinically confirmed when angioplasty and stenting of the iliac veins are accompanied by severe pain.
Another interesting fact. Venous pain does not occur if the lumen of the vein is expanded using pharmacological agents, for example, by applying adenosine [8]. In other words, dilatation of healthy veins cannot be the cause of pain. This hypothesis is confirmed by the absence of pain in patients with an arteriovenous fistula for hemodialysis.
Numerous epidemiological studies indicate that there is no correlation between the severity of symptoms and the severity of clinical manifestations of CVD. Although, in the Edinburgh epidemiological study in women, a weak connection was established between varicose veins of the main saphenous veins and a number of symptoms (pain, heaviness and itching), nevertheless, 40% of women had severe varicose veins in the complete absence of physical complaints. In contrast, 45% of patients with typical venous pain had no clinical signs of CVD.
Interestingly, in men there was no connection between pain and the severity of varicose veins [9].
What causes venous pain?
Currently, it is associated with an inflammatory reaction caused by phlebostasis. Inflammation of the venous wall not only causes pain, but also acts as a key mechanism leading to varicose transformation with qualitative and quantitative damage to the cellular and tissue components of the venous wall. The trigger for this mechanism may be regional hypoxia caused by capillary stasis. This confirms a significant decrease in the partial oxygen tension in the vessels of the microvasculature after patients with CVD remained in immobile orthostasis for 30 minutes [10].
In addition, it has been proven that it is hypoxia in the microvasculature that causes activation of endothelial cells, which is manifested by an increase in the concentration of calcium in their cytoplasm, which in turn leads to an increase in the activity of phospholipase A2. Activation of phospholipase A2 is one of the key mechanisms for the synthesis of inflammatory mediators such as bradykinin, prostaglandins E2 and D2, platelet activating factor (PAF) and leukotriene B4. PAF increases local serotonin and histamine activity, causing increased adhesion of neutrophils to the venous endothelium. This process subsequently leads to the infiltration of the venous wall by active neutrophils and their stimulation of the synthesis of leukotriene B4.
Evidence of damage to the venous wall as a result of the inflammatory response, in which neutrophils and endothelial cells are the main participants, has been accumulating over the past 5 years. The presence of neutrophils, monocytes, activated T-lymphocytes, accumulation of macrophages and mast cells, expression of cell adhesion molecules on the surface of leukocytes and endothelial cells (LFA-1, VLA-4, ELAM-1, ICAM-1, VCAM-1), synthesis of cytokines ( IL-1 beta, IL-6, TNF alpha) and prothrombotic factors (von Willebrandt) serve as objective criteria for the inflammatory process in the vein wall.
Many proinflammatory mediators synthesized as a result of hypoxia can act as algogens that activate venous and paravasal nociceptors. In particular, intravenous or paravasal administration of bradykin causes pain in healthy people. A number of studies show that the algogenic effect of bradykinin depends on the synthesis of NO by endothelial and smooth muscle cells of the venous wall, followed by activation of cGMP synthesis [11]. The pain effect of bradykinin is potentiated by prostaglandin E2, which, although itself not an algogen, increases the sensitivity of nociceptors.
In other words, the generation of venous pain occurs as a result of self-accelerating cascade reactions, with the participation of various substances (a kind of “inflammatory mixture”), which not only activate nociceptors, but also disrupt vascular permeability, leading to plasma extravasation with the development of transmural and tissue edema. Over time, this process causes damage to the venous wall with disintegration of its structure and loss of normal elastic properties.
Indirect confirmation of the presented hypothesis is the increase in NO levels and markers of endothelial dysfunction as CVD progresses. Here, it is logical to assume that the severity of inflammation in the vein wall, and, consequently, the level of inflammatory markers will directly correlate with the intensity of the pain syndrome and the degree of varicose transformation.
Meanwhile, this hypothesis was not confirmed in a clinical study, when blood samples were taken from a vein in the dorsum of the foot from 132 patients (C2-C5 according to CEAP) and the level of 12 inflammatory markers was correlated with the intensity of pain assessed on a visual analogue scale [12] . Moreover, it turned out that the pain syndrome does not correlate with the severity of varicose transformation of the saphenous veins.
Thus, there must be some pathophysiological mechanism that induces pain. This can be regional hypoxia, which is provoked by a variety of situations. Indeed, venous pain and other nociceptive disorders usually appear at the end of the working day, after prolonged periods of sitting or standing, as well as during certain phases of the menstrual cycle. Moreover, all these symptoms can occur, not only against the background of visible pathomorphological changes in the venous system in patients with CVD.
In other words, a cascade of chemical reactions that activate venous and paravasal nociceptors is formed long before the pathological remodeling of the venous wall. This position is confirmed by the higher frequency of subjective complaints, including venous pain and heaviness in the legs in patients in the absence of both varicose veins and pathological venovenous reflux. Thus, venous pain and varicose transformation of the venous wall are based on similar biochemical and molecular cellular mechanisms, in particular inflammation. At the same time, the time period required for the development of varicose transformation of peripheral veins, as a rule, is significantly longer in comparison with pain and other nociceptive manifestations in patients with CVD.
How can we explain the significant reduction in pain and other symptoms in the later stages of CVD?
Apparently, this is due to damage to subendothelial and paravasal nociceptors. Damage to sensory nerve endings reflects peripheral neuropathy associated with venous microangiopathy and increased endoneurial pressure. This is confirmed by the fact that in the later stages of CVD the threshold for tactile, vibration and temperature sensitivity increases significantly, which can be explained by the loss of sensory axons. Interestingly, the sensory threshold increases significantly in patients with C5 compared with C2 class CVD [13].
Thus, the decrease in pain in the later stages of the disease can be explained by a decrease in the number of nociceptors.
Pain syndrome is also determined by other components, depending on the individual characteristics of the patient. Such options would include both the reactivity of cellular components (endothelial cells, neutrophils, venous and paravasal nociceptors) and the mechanisms of integration of nociceptive stimuli in the brain. In particular, the amount of different prostaglandins synthesized by endothelial cells is determined by at least 10 individual factors. Similarly, neutrophil activity depends on age and previous sensitivity to various inflammatory signals. In addition, the density of venous and paravenous innervation, as well as the density of ion nociceptive channels that transform a chemical signal into a nerve impulse, has individual characteristics. The resulting chain of these algogenic processes is modulation in the brain, which in turn depends on the effect of endogenous opioids, which vary and depend on genetic factors. An example is the genotype of the enzyme catechol-0-methyl-transferase, which determines the amount of endogenous opioids during a pain impulse and significantly influences pain sensitivity in healthy people [14].
The search for a correlation between the severity of venous pain and other subjective symptoms with the nature of changes in the peripheral venous bed and other factors in patients with CVD is hampered by both the lack of special validated questionnaires and sensitive instrumental methods. In addition, the presence of diverse and poorly controlled endogenous and exogenous factors hinders the study of the exact mechanisms of venous pain and other symptoms of CVD. That is why, the main searches are carried out in experimental studies of the interaction between inflammatory mediators and nociceptors [15,16].
Treatment of venous pain and other symptoms of chronic venous diseases.
Treatment of venous pain and other nociceptive symptoms associated with CVD involves a preliminary objective assessment of not only the state of the venous system of the lower extremities, but also the general clinical status, including psychological, behavioral, and, if necessary, neurophysiological characteristics. In some cases, it is advisable to involve specialized specialists: neurologists and psychoneurologists.
In general, the treatment of symptoms and syndromes that occur with chronic venous disease fits into the general concept of pain syndrome therapy, which includes:
- Elimination of the source of pain and restoration of damaged tissues;
- Impact on peripheral components of pain: elimination of inflammation and swelling, suppression of the synthesis of prostaglandins and other algogens;
- Inhibition of pain impulses along peripheral nerves;
- Impact on processes occurring in the dorsal horns of the spinal cord;
- Impact on the psychological (and at the same time neurochemical) components of pain using pharmacological drugs and psychotherapeutic methods.
The most important component of the pathogenetic treatment of venous pain is compression therapy using bandages or medical knitwear. Its use makes it possible to improve venous blood circulation and microcirculation in the shortest possible time, thereby reducing the degree of excitation of intravenous and paravasal nociceptors.
The prescription of phlebotropic drugs (synonyms: venotonics, phleboprotectors) deserves special discussion. Currently, they are considered as a first-line treatment for the treatment of symptomatic forms of CVD [17,18]. As already discussed above, the most striking nociceptive symptoms appear in the earliest stages of the formation of CVD, when there are no significant disturbances in the venous circulation, and the pathological process affects mainly the microvasculature. Under these conditions, the main task is to suppress the leukocyte-endothelial reaction and its consequences in the form of uncontrolled synthesis of various biologically active substances, many of which act as algogens [19,20].
How do phlebotropic drugs affect venous pain?
RELIEF was one of the first studies where, on a large clinical material (3132 patients, C0S-C4) using a specialized questionnaire (CIVIQ) and a visual analogue scale (VAS), a significant reduction in pain and other symptoms caused by both organic (valvular insufficiency) and ), and functional (phlebopathy) damage to the venous system of the lower extremities [21].
Of interest are the results of a recent double-blind, placebo-controlled study of 592 patients (C3-C4A) with superficial (34%), deep or perforating vein reflux (66%) who had venous pain initially assessed as greater than 4 cm YOUR. Patients in the main group received 2 tablets of Detralex per day, while in the control group - 2 tablets of placebo. Control endpoints were a decrease in pain by at least 3 cm on the VAS scale and an increase in quality of life by at least 20 points on the CIVIQ scale. These results were achieved in 24.6% of patients receiving Detralex and 14.8% of the placebo group (RR=1.67) [22].
What is the optimal duration of pharmacotherapy for pain and other symptoms caused by CVD? To some extent, the answer to this question is provided by a multicenter study by Guillot et al., who recorded a significant decrease in CVD symptoms assessed by a doctor using VAS (values from 0 to 5) every 2 months. All patients took 2 Detralex tablets daily for 12 months. According to the results of the study, a maximum decrease (by approximately 50%) in the severity of CVD symptoms was noted in the first two months. Subsequently, this trend, although less pronounced, persisted, which was noted at each clinical examination [23].
In a high-profile meta-analysis of 5 randomized trials examining the treatment of patients with C6 (open trophic ulcers), Coleridge-Smith et al. [24] showed that the inclusion of Detralex not only promotes faster closure of venous trophic ulcers with an area of up to 10 cm2, but also significantly reduces the severity of pain.
In the context of the prevention of pain in patients with CVD, two more works deserve attention, devoted to the analysis of the features of the perioperative period in patients with C2 who received Detralex. Thus, Veverkova et al. [25] demonstrated a significant reduction in the amount of analgesics required for postoperative pain relief after safenectomy in patients receiving Detralex. Similar data were obtained during the Russian multicenter study DEFANCE [26,27].
Concluding this review, it should be noted that many controversial issues remain regarding both the pathogenesis of chronic venous diseases and methods of their treatment. Only a number of key provisions are beyond doubt:
- Treatment of CVD must begin as early as possible, even when there are no signs of organic damage to the venous bed, but symptoms characteristic of this disease are present.
- It is advisable to use a set of measures, including both conservative methods (normalization of lifestyle, compression, pharmacotherapy, etc.) and surgical interventions, the volume of which should be exactly commensurate with the nature and extent of damage to the venous bed.
- Like any chronic disease, venous insufficiency, regardless of its genesis, tends to recur. That is why all patients with CVD, regardless of whether they have been operated on or not, require lifelong monitoring with, if necessary, therapeutic and preventive measures.
Literature:
- Eklof Bo, Perrin M., Konstantinos T. Delis et al. Update terminology of chronic venous disorders: The VEIN-TERM transatlantic interdisciplinary consensus document. J Vasc Surg 2009; 49:498-501.
- Labropoulos N., Stansby G. Venous and Lymphatic Diseases. Taylor & Francis.2006; 559.
- Ruckley CV, Fowkes FGR, Bradbury AW Venous Disease. Epidemiology, Management and Delivery of Care. Springer.1999; 278.
- Jawien A., Grzela T., Ochwat A. Prevalence of chronic venous insufficiency in men and women in Poland: multicentre cross-sectional study in 40095 patients. Phlebology. 2003; 81: 110-122.
- Langer RD, Ho E, Deneberg JO, et al. Relationship between symptoms and venous disease. Arch Int Med 2005;165:1420-1424.
- Arndt JO, Klement W. Pain evoked by polymodal stimulation of hand veins in humans. J Physiol 1991;440:467.
- Klement W., Arndt JO Pain but no sensation temperature are evoked by termal stimulation of cutaneous veins in man. Neurosci Lett.1991;123:119-122.
- Klement W., Arndt JO Adenosine does not evoke pain from venous and paravascular nociceptors in the human. Cardivasc Res 1992;26:186-189.
- Bradbury A., Evans C., Allan P., et al. What are the symptoms of varicose veins? Edinburgh vein study cross sectional population survey. BMJ.1999;318:353-356.
- Badier-Commandier C., Jacob MP, Michel JB Le remodelage variqueux. Medecine Therapeutique.2006;6:718-723.
- Danziger N. Pathophysiology of pain in venous disease. Phlebolymphology.2008; 15: 107-114
- Howlader MH, Smith PD Symptoms of chronic venous disease and association with systemic inflammatory markers. J Vasc Surg 2003;38:950-954.
- Padberg FT Jr., Maniker AH, Carmel G., et al. Sensory Impairment: a feature of chronic venous insufficiency. J Vasc Surg 1999;30:836-842.
- Zubieta JK, Heitzeg MM, Smith YR, et al. COMT vall58met genotype affects mu-opioid neurotransmitter responses to a pain stressor. Science.2003;299:1240-1243.
- Ramelet AA, Boisseau MR, Allegra C.et all. Veno-active drugs in the management of chronic venous disease. An international consensus statement: Current medical position, prospective views and final resolution. Clinical Hemorheology and Microcirculation. 2005; 33: 309-319
- Bergan J., Scmid-Schonbein G., Coleridge-Smith Ph., et al. Chronic Venous Disease, N Engl J Med. 2006; 355:488-498
- Schmid-Schonbein GW, Granger DN Molecular Basis for Microcirculatory Disorders. Springer. 2003; 640
- Bergan J., Shortell C. Venous Ulcers. Elsevier. 2007; 341
- Nicolaides AN, Allegra C., Bergan J. et all. Management of Chronic Venous Disorders of the Lower Limbs Guidelines According to Scientific Evidence. International Angiology. 2008; 27: 1-59
- Ramelet A.A., Perrin M., Kern P., Bounameaux H. Phlebology, 5th edition, Elsevier Masson. 2008: 565
- Jantet G. RELIEF Study Group. Chronic Venous Insufficiency: Worldwide Results of the RELIEF Study. Angiology. 2002; 53: 245-256
- Pitsch F. Assessment of treatment efficacy on venous symptoms: the example of Detralex. Phlebolymphology.2008; 15: 137-142.
- Guillot B., Guilhou JJ, de Champvallins M., et al. A long term treatment with a venotropic drug: results on efficacy and safety on Daflon 500 mg in chronic venous insufficiency. Int Angiol. 1989; 8: s67-s71.
- Colerige-Smith P., Lok C., Ramelet A.A. Venous leg ulcer: meta-analysis of adjunctive therapy with micronized purified flavonoid fraction. Eur J Vasc Endovasc Surg. 2005; 30: 198-208
- Veverkova L., Jedika V., Wechsler J., et al. Analysis of the various procedures used in great saphenous vein surgery in the Czech Republic and benefit of Daflon 500 mg on postoperative symptoms. Phlebolymphology.2006;13: 195-201
- Savelyev V.S., Pokrovsky A.V., Kirienko A.I., et al. Surgical intervention for varicose veins “under the cover” of micronized diosmin. Angiology and vascular surgery.-2007; 13:47-55
- Saveljev V., Pokrovsky A., Kirienko A., et all. Striping of the great saphenous vein under micronized purified flavonoid fraction (MPFF) protection (results of the Russian multicenter controlled trial DEFANCE). Phlebolymphology. 2008; 15:43-51
Do gels, decoctions, tablets and ointments help against varicose veins?
It is much easier and cheaper to take a pill, take infusions or smear inflamed veins, but such effective miracle remedies have not yet been invented, and long-term results testify against this, but self-hypnosis can work a miracle. Whether to throw money away is up to you. Selling medications for varicose veins on the legs has become a profitable business for pharmaceutical companies, since varicose veins are a very common disease.
Remedies for varicose veins are always of interest to suggestible patients, and since the veins do not bother them in the initial stages of the disease, patients are wary of any surgical help. Pharmaceutical companies spend enormous amounts of money to convince the population and even doctors that their pills are the best for varicose veins. The Servier company has been especially successful in this, organizing conferences and “research”, buying famous phlebologists in order to gain their authority to increase sales of its pills.
There are also folk remedies for “getting rid” of varicose veins:
Soda, mumiyo, iodine, peroxide, soap, clay, onion, cabbage, propolis and other remedies for varicose veins in the most miraculous way allow you to get rid of varicose nodes, mesh, as well as kidney and gallstones, tumors and gangrene. Ordinary people believe in this nonsense, not understanding either the causes or consequences of the disease. Thousands of messages on the Internet about magic pills and remedies for varicose veins on the legs are causing confusion in the minds of patients.