pharmachologic effect
Lozap is an antihypertensive drug. Its effect is based on preventing the binding of angiotensin 2 to AT1 receptors. As a result, a number of AT2 effects are leveled out:
- arterial hypertension;
- release of renin and aldosterone , vasopressin , catecholamines ;
- development of left ventricular hypertrophy .
Under the influence of the drug, the angiotensin-converting enzyme is not blocked, so bradykinin does not accumulate and the kinin system is not affected.
The active metabolite Lozap is formed during biotransformation and has an antihypertensive effect on the body. Therefore, the drug is a prodrug.
The drug has the same effect on patients, regardless of their age, race and gender.
Pharmacological properties of the drug Lozap
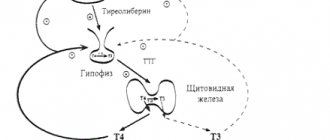
Losartan is a specific antagonist of the receptor (AT1 type) of angiotensin II. Angiotensin II binds to the AT1 receptor, which is found in many tissues (eg vascular smooth muscle, adrenal glands, kidneys and heart). Losartan and its pharmacologically active metabolite carboxylic acid (E-3174) block the effects of angiotensin II. Losartan does not bind or block other hormone receptors and ion channels important for cardiovascular regulation. Losartan does not inhibit ACE, but blocks the response to angiotensin I and II and has no effect on the kinin system or bradykinin levels. Losartan has no effect on autonomic reflexes and the level of norepinephrine in the blood plasma. The drug is equally effective in men, women under 65 years of age and in elderly and senile patients with hypertension (arterial hypertension). With simultaneous use of Lozap with thiazide-type diuretics, the degree of blood pressure reduction increases. After oral administration, losartan is well absorbed and, as a result of metabolism during the initial passage through the liver, an active metabolite of carboxylic acid and inactive metabolites are formed. Systemic bioavailability after oral administration of losartan is about 33%. The average maximum concentration of losartan is achieved within 1 hour, and its active metabolite - within 3-4 hours. When using the drug with a standardized diet, no clinically significant effect on the profile of the concentration of losartan in the blood plasma was noted. Plasma clearance for losartan and its active metabolite is approximately 600 and 50 ml/min, respectively. The renal clearance of losartan and its active metabolite is about 74 and 26 ml/min, respectively. After oral administration, almost 4% of the dose is excreted unchanged in the urine and about 6% of the dose as an active metabolite. With oral administration of losartan potassium in doses up to 200 mg, the pharmacokinetics of the drug and its active metabolite are linear. After oral administration, the concentration of the drug and its active metabolite in the blood plasma decreases polyexponentially with a final half-life of 2 hours for losartan and 6–9 hours for the active metabolite. At a single daily dose of 100 mg, neither losartan nor its active metabolite accumulates in the blood plasma in significant quantities.
Pharmacodynamics and pharmacokinetics
Lozap is an antihypertensive drug, a specific angiotensin II receptor antagonist. It reduces peripheral vascular resistance, reduces the level of adrenaline and aldosterone in the blood. Under its influence, the pressure in the pulmonary circulation decreases, a diuretic effect develops, and afterload decreases. Lozap prevents myocardial hypertrophic processes and helps increase tolerance to physical activity in people suffering from heart failure.
The maximum hypotensive effect after a single dose of the drug is observed after 6 hours, after which it gradually decreases over 24 hours. With systematic treatment, the maximum effect (lowering blood pressure) occurs three to six weeks after the start of therapy.
It should be taken into account that in people who have cirrhosis of the liver losartan increases markedly . Therefore, such patients are prescribed a special, reduced dosage.
Absorption from the human gastrointestinal tract occurs quickly. The bioavailability of the drug is about 33%. After oral administration, the highest plasma concentration is present after one hour. The highest concentration of the drug metabolite is observed after 3-4 hours. The half-life of losartan is 2 hours, the active metabolite is 9 hours. 35% of the drug is excreted from the body in the urine, about 60% through the intestines.
Lozap, 50 mg, film-coated tablets, 30 pcs.
Hypersensitivity.
Patients with a history of angioedema (swelling of the face, lips, pharynx and/or tongue) should be closely monitored.
Arterial hypotension and water-electrolyte imbalance.
Symptomatic hypotension, especially after the first dose or after dose increases, may occur in patients with hypovolemia and/or hyponatremia as a result of high-dose diuretics, a low-salt diet, diarrhea, or vomiting.
It is necessary to either correct these conditions before prescribing Lozap®, or use initial doses of the drug.
Water and electrolyte disturbances.
Water and electrolyte disturbances are typical for patients with impaired renal function in combination with or without diabetes mellitus and require correction. In a clinical study conducted in type 2 diabetic patients with nephropathy, the incidence of hyperkalemia in the losartan group was higher than in the placebo group. This indicates the need for constant monitoring of potassium levels in the blood plasma and creatinine Cl levels - patients with heart failure and creatinine Cl levels from 30 to 50 ml/min require especially strict monitoring.
Prescribing potassium-sparing diuretics, potassium supplements and potassium-containing salt substitutes simultaneously with Lozap® is not recommended.
Liver dysfunction.
Taking into account pharmacokinetic data indicating a significant increase in plasma concentrations of losartan in patients with cirrhosis, patients with a history of impaired liver function (more than 9 points on the Child-Pugh scale) are recommended to prescribe the drug in lower doses. There is no experience with the use of the drug in patients with severe liver failure. Taking this into account, Lozap® is contraindicated in patients with severe liver failure.
Double blockade of the RAAS.
There is evidence that the simultaneous use of ACE inhibitors, ARB II or aliskiren increases the risk of arterial hypotension, hyperkalemia and renal dysfunction (including acute renal failure).
The use of Lozap® in combination with aliskiren is contraindicated in patients with diabetes mellitus and/or moderate or severe renal failure (GFR <60 ml/min/1.73 m2) and is not recommended in other patients (see “Contraindications”).
The use of Lozap® in combination with an ACE inhibitor is contraindicated in patients with diabetic nephropathy and is not recommended for other patients (see “Contraindications”).
IHD and cerebrovascular diseases.
As with the use of any antihypertensive drugs, too sharp a decrease in blood pressure in patients with coronary artery disease and cerebrovascular diseases can lead to myocardial infarction or ischemic stroke.
Heart failure.
In patients with heart failure with or without renal impairment, as with other drugs acting on the RAAS, there is a risk of developing severe hypotension and acute renal failure.
experience with the use of losartan in the treatment of patients with heart failure and concomitant severe renal failure in patients with severe CHF (functional class IV according to the NYHA
), as well as in patients with heart failure and life-threatening arrhythmias. With this in mind, caution should be exercised when prescribing losartan to these categories of patients.
Combined use with ACE inhibitors for CHF.
When using Lozap® in combination with ACE inhibitors, the risk of side effects may increase, especially renal dysfunction and hyperkalemia (see “Side Effects”). In these cases, careful observation and monitoring of laboratory parameters is necessary.
Hemodialysis.
During hemodialysis, the sensitivity of blood pressure to the action of AT1 receptor antagonists increases as a result of a decrease in blood volume and activation of the RAAS. It is necessary to adjust the dose of Lozap® under careful monitoring of blood pressure in patients on hemodialysis.
Kidney transplantation.
There are no data on the use of Lozap® in patients who have recently undergone a kidney transplant.
General anesthesia.
Patients receiving ARA II during general anesthesia and surgical procedures may develop arterial hypotension as a result of blockade of the RAAS. Very rarely, cases of severe arterial hypotension may occur, requiring IV fluids and/or vasopressors.
Aortic and mitral valve stenosis, hypertrophic obstructive cardiomyopathy.
When using the drug Lozap®, as well as other vasodilators, caution should be exercised in patients with hypertrophic obstructive cardiomyopathy or hemodynamically significant stenosis of the aortic or mitral valve.
Primary hyperaldosteronism.
Patients with primary hyperaldosteronism are usually resistant to treatment with antihypertensive drugs that affect the RAAS. In this regard, Lozap® is not recommended for use in such patients.
Elderly patients.
As a rule, patients over 75 years of age are recommended to start treatment with Lozap® with a dose of 25 mg/day.
Other special instructions and precautions.
As clinical experience with the use of ACE inhibitors, losartan and other AT1 receptor antagonists shows, these drugs are less effective in reducing blood pressure in patients of the Negroid race than in representatives of other races, possibly due to low renin activity in patients of this race.
Impact on the ability to drive vehicles and machinery.
Not studied. When driving vehicles and engaging in potentially hazardous activities that require increased concentration and speed of psychomotor reactions, it should be taken into account that when using the drug, dizziness, drowsiness and fainting may occur.
Indications for use
Lozap is used in the following cases:
- arterial hypertension disease ;
- chronic heart failure (the drug is included in combination treatment, used to treat patients with intolerance to ACE inhibitors, or when their ineffectiveness is noted);
- the need to reduce the risk of cardiovascular diseases (including stroke ) in people suffering from arterial hypertension and left ventricular hypertrophy ;
- development of diabetic nephropathy in patients with type 2 diabetes mellitus
Indications for use of Lozap Plus are the same. However, it has a combined composition, so indications for the use of the drug Lozap Plus include conditions for arterial hypertension, which require additional treatment with diuretics.
Lozap indication
The use of Lozap is relevant for the following conditions:
- High blood pressure.
- Chronic course of heart failure.
- Prevention of the development of severe vascular and cardiac diseases among patients with hypertension and left ventricular enlargement.
- Nephropathy and high blood pressure in people with diabetes.
- Contraindications
- Absolute contraindications for use are:
- Increase in potassium levels in the blood.
- Low pressure.
- Individual intolerance to the medicinal components included in the composition.
- The time of bearing a child.
- Breastfeeding.
- Symptoms of dehydration.
- Children under 18 years of age.
Side effects
Most often, no side effects are observed during treatment with the drug. If side effects develop, their nature is transient, so discontinuation of the drug is usually not required. Rarely, the following may be a concern:
- Functions of the sensory organs and nervous system: fatigue, headaches, dizziness , insomnia, asthenia. In less than 1% of cases, drowsiness, anxiety, memory loss, depression , paresthesia , tremor , hypoesthesia , migraine , hearing, vision, and taste develop.
- Functions of the respiratory system: symptoms of upper respiratory tract infections. bronchitis , dyspnea , and rhinitis develop .
- Functions of the digestive system: abdominal pain, nausea, diarrhea . In less than 1% of cases, dry mouth, gastritis , vomiting, toothache, flatulence , and constipation are .
- Functions of the musculoskeletal system: pain in the back, limbs, chest; convulsions, myalgia. In less than 1% of cases, arthritis , arthralgia, and pain in the shoulder and knee are noted.
- Functions of the cardiovascular system: dose-dependent hypotension, tachycardia or bradycardia , angina pectoris , arrhythmia , anemia .
- Functions of the genitourinary system: in less than 1% of cases, the drug negatively affects potency, urinary tract infections and impaired renal function are observed.
- Dry skin, increased sweating, allergic reactions, and hyperkalemia are also rarely observed.
Side effects of the drug Lozap
May occur in the form of anaphylactic reactions, angioedema, including swelling of the larynx and glottis, with the development of airway obstruction and/or swelling of the face, lips, pharynx and/or tongue. In controlled clinical trials, the only side effects were dizziness and hypotension. In some cases, patients receiving losartan experienced hepatitis, diarrhea, impaired liver function, migraine, myalgia, arthralgia, urticaria, and itching. Adverse reactions from the respiratory tract have been described: cough, upper respiratory tract infections. When treated with losartan, a dry cough is detected.
Instructions for use of Lozap (Method and dosage)
The drug is taken orally, there is no dependence on food intake. The tablets should be taken once a day. Patients with arterial hypertension take the medicine 50 mg per day. To achieve a more noticeable effect, the dose is sometimes increased to 100 mg. How to take Lozap in this case, the doctor gives recommendations individually.
The instructions for Lozap stipulate that patients with heart failure take the medicine 12.5 mg once a day. Gradually, the dose of the drug is doubled at intervals of one week until it reaches 50 mg once a day.
Instructions for use of Lozap Plus include taking one tablet once a day. The largest dose of the drug is 2 tablets per day.
If a person is simultaneously taking high doses of diuretics, the daily dose of Lozap is reduced to 25 mg.
Elderly people and patients with impaired renal function (including those on hemodialysis) do not need to adjust the dosage.
Composition and release form
The drug Lozap is available in the form of small tablets for parenteral administration. They are white, slightly elongated and convex on both sides. They have a soluble thin film shell. Packaged in blisters of 10 pieces. One cardboard package contains 3, 6, 9 blisters and includes instructions for use.
The active substance is potassium losartran.
Auxiliary components: cellulose, povidone, talc, magnesium stearate, macrogol, dyes, mannitol, croscarmellose sodium.
Interaction
Taking the drug simultaneously with Rifampicin and Fluconazole leads to a decrease in the content of the active metabolite in the blood plasma.
It should be borne in mind that Lozap may enhance the effects of other drugs with an antihypertensive effect: adrenergic blockers, diuretics, ACE inhibitors. Patients with dehydration resulting from treatment with large doses of diuretics may notice a marked decrease in blood pressure after taking Lozap.
In case of simultaneous use with potassium preparations, potassium-sparing diuretics, potassium levels must be constantly monitored, as there is a risk of developing hyperkalemia .
The hypotensive effect of the drug decreases after taking Indomethacin and other NSAIDs.
clinically significant interactions with hydrochlorothiazide , Digoxin , Cimetidine , Warfarin , Phenobarbital and Erythromycin .
Interactions with drugs
Combining the drug with Fluconazole and Rifampicin leads to a decrease in the active substance.
Lozap increases the pharmacological activity of other antihypertensive drugs.
It is not recommended to take the medicine in parallel with non-steroidal anti-inflammatory drugs.
When interacting with potassium and potassium-sparing diuretics, it is important to monitor the level of the element in the blood to prevent the occurrence of hyperkalemia.
Patients with obvious symptoms of dehydration while taking diuretics may experience a rapid decrease in blood pressure when taking an antihypertensive drug.
special instructions
The blood pressure medicine Lozap is prescribed with caution to people who have previously received large doses of diuretics.
Before taking blood pressure pills, it is necessary to rule out liver disease. Patients with liver pathologies should take lozap high blood pressure tablets in reduced doses.
The drug is prescribed with caution to people suffering from stenosis of the artery of a single kidney , as well as bilateral stenosis of the renal arteries .
Under the influence of the drug, the ability to control transport and interact with complex mechanisms is retained.
Lozap overdose, symptoms and treatment
In case of overdose (intoxication) of the drug, symptoms such as hypotension, tachycardia may appear, and sometimes bradycardia may occur due to parasympathetic (vagal) stimulation. If symptomatic hypotension occurs, maintenance therapy is provided. In case of accidental overdose (taking the drug in a high dose), it is necessary to carry out symptomatic and supportive therapy, induce vomiting and gastric lavage. Neither losartan nor its active metabolite can be removed by hemodialysis.
Analogs
Level 4 ATC code matches:
Telmisartan
Irbesartan
Presartan
Nortivan
Candesartan
Kozaar
Aprovel
Teveten
Blocktran
Cardosal
Valsartan
Losartan
Atakand
Diovan
Valsacor
Mikardis
Vazar
Valz
Lorista
Lorista
Pharmacies offer analogues of Lozap from different manufacturers. At the same time, the price of analogues is both lower and higher compared to the cost of this drug. Patients should remember that this medicine and its analogues can affect the body in different ways, therefore, if you need to purchase analogues of Lozap Plus or Lozap, you should first consult with your doctor.
Analogues that contain a similar active ingredient are the following drugs:
- Kozaar
- Presartan
- Sentor
- Losartan
- Lakea
- Lorista
- Losarel
Analogues have the same indications for use, differ in dosage, manufacturer, and cost.
Lozap or Lorista – which is better?
The active ingredient in Lorista is the same as in Lozap. Lorista is prescribed to patients suffering from arterial hypertension and chronic heart failure. At the same time, the cost of Lorista is lower. However, the analogue can be used only after consultation with a doctor and after the annotation has been carefully read.
What is the difference between Lozap and Lozap Plus?
If it is necessary to undergo a course of treatment with this drug, the question often arises, which is better - Lozap or Lozap Plus? When choosing a drug, you should take into account that Lozap Plus combines losartan and hydrochlorothiazide, which is a diuretic and has a diuretic effect on the body. Therefore, these tablets are indicated for those patients who require combination therapy.
Reviews
Reviews of Lozap Plus and Lozap indicate that in most cases the drugs effectively lower blood pressure and have a positive effect on the health of people with diseases of the cardiovascular system.
Patients who go to a specialized forum to leave reviews on Lozap 50 mg note that coughing, dry mouth, and hearing impairment are sometimes noted as side effects. But in general, patient reviews of the medicine are positive. At the same time, reviews from doctors indicate that the drug may not be suitable for all people suffering from arterial hypertension. Therefore, initially it should be taken under the strict supervision of a specialist.
Price, where to buy
Price for Lozap 50 mg, 30 pcs. - about 400 rubles, price for Lozap 100 mg, 30 pcs. - about 500 rubles.
- Online pharmacies in RussiaRussia
- Online pharmacies in UkraineUkraine
- Online pharmacies in KazakhstanKazakhstan
Pharmacy Dialogue
- Lozap AM tablets p/5mg+50mg No. 30Hanmi Pharm
RUB 442 order - Lozap AM tablets p/5mg+100mg No. 30Hanmi Pharm
RUB 462 order
- Lozap (tab.p.pl/vol. 100 mg No. 30) Saneca
RUB 356 order
show more
Pharmacy24
- Lozap 50 mg No. 90 tablets AT Saneka Pharmaceuticals, Slovak Republic/Zentiva LLC Czech Republic
178 UAH order - Lozap 50 mg No. 30 tablets AT Saneka Pharmaceuticals, Slovak Republic/Zentiva LLC Czech Republic
100 UAH order
- Lozap 100 plus 100mg/25mg N90 tablets Sanofi-Aventis S.p z o.o., Poland
314 UAH. order
- Lozap 100 mg No. 30 tablets AT Saneka Pharmaceuticals, Slovak Republic/Zentiva LLC Czech Republic
111 UAH order
- Lozap 100 mg No. 90 tablets AT Saneka Pharmaceuticals, Slovak Republic/Zentiva LLC Czech Republic
263 UAH order
PaniPharmacy
- Lozap plus tablets Lozap plus tablets. p/o No. 90, Zentiva
276 UAH. order
- Lozap tablets Lozap tablets. p/o 100 mg No. 30, Saneca Pharmaceuticals
130 UAH order
- Lozap tablets Lozap tablets. p/o 50 mg No. 90, Saneca Pharmaceuticals
198 UAH order
- Lozap tablets Lozap tablets. 100 mg No. 90, Saneca Pharmaceuticals
277 UAH order
- Lozap plus tablets Lozap plus tablets. p/o No. 30, Zentiva
145 UAH order
show more