Use during pregnancy and breastfeeding:
The safety of Mildronate during pregnancy has not been proven. To avoid possible adverse effects on the fetus, Mildronate should not be prescribed during pregnancy. Currently, there are data from clinical studies on the use of Mildronate as part of a combination therapy for the regulation of discoordinated labor, as well as as part of a complex treatment of placental insufficiency for the purpose of preventing and treating functional disorders of the development of the central nervous system in the fetus.
It is not known whether Mildronate is excreted in breast milk. If it is necessary to use Mildronate during lactation, breastfeeding should be discontinued.
Side effects:
From the cardiovascular system: tachycardia, hypotension.
From the side of the central nervous system: psychomotor agitation.
From the digestive system: dyspeptic symptoms.
Allergic reactions: redness, rashes, itching, swelling.
Special instructions and precautions:
Patients with chronic liver and kidney diseases should use caution with long-term use of Mildronate.
The use of Mildronate as part of combination therapy for patients with chronic heart failure increases exercise tolerance.
For myocardial infarction, Mildronate is not a first-line drug.
Clinical studies have revealed a beneficial effect of Mildronate on the state of lipid metabolism (decreasing the content of atherogenic lipids in the blood).
It should be kept in mind that 1 scoop (5 ml) of Mildronate syrup contains 2.75 g of sorbitol and 0.25 g of glycerin.
There is insufficient data on the safety and effectiveness of Mildronate in children under 12 years of age.
There is no evidence of an adverse effect of Mildronate on the reaction rate.
Drug interactions:
Mildronate can be combined with antianginal drugs, anticoagulants, antiplatelet agents, antiarrhythmic drugs, cardiac glycosides, diuretics, bronchodilators.
Since Mildronate may cause mild tachycardia and a decrease in blood pressure, caution should be exercised when combining Mildronate with antihypertensive agents and peripheral vasodilators.
Storage conditions:
Store in a dry place, out of reach of children, at a temperature not exceeding 25°C.
Despite the successes achieved in the treatment of patients with cardiovascular diseases (CVD), they still remain the main cause of mortality and disability in the population. According to epidemiological data, in Russia, 74.9% of men and 72.2% of women aged 25-64 years die from coronary heart disease (CHD) and cerebrovascular diseases [1].
The basic therapy for IHD is drugs with antiplatelet, hypolipidemic, as well as hemodynamic and neurohormonal effects, which optimize the relationship between myocardial oxygen needs and its delivery. However, the possibilities of therapy in this direction are limited by the conditions of myocardial functioning during ischemia, the presence of other adaptive and maladaptive processes that have a significant impact on cardiomyocytes (CMC) and the myocardium as a whole. In addition, in the early post-infarction period (EPI), ischemic myocardial remodeling develops, which serves as a powerful predictor of cardiovascular death [2]. The triggering mechanism for post-infarction remodeling is the loss of CMC, which leads to the emergence of conditions conducive to the development of myocardial damage in the border areas and areas remote from the lesion [3]. Cardiac remodeling in the early period after myocardial infarction (MI) can be of a different nature, but an important role in this process is played by increased activity of the sympathetic-adrenal (SAS) and renin-angiotensin systems, activation of the local and systemic inflammatory response [4]. Ischemic remodeling during this period is a dynamic, reversible process. Of course, the main method of its prevention is timely reperfusion of the myocardium. At the same time, understanding the essence of the disorders occurring in the metabolism of CMC during myocardial infarction and the description of new adaptive ischemic syndromes (stupefaction, myocardial hibernation) [5] open up the possibility of a new direction of drug influence on the ischemic myocardium during this period - myocardial cytoprotection.
The high anti-ischemic effectiveness of one of the myocardial cytoprotectors - meldonium - has been demonstrated in a number of recent studies in angina pectoris (MILSS, MILSS I, MILSS II studies), its positive effect on exercise tolerance, a decrease in the clinical manifestations of angina pectoris, a decrease in the need for nitrates, including including in elderly patients. However, a comprehensive study of the effect of meldonium with short-term parenteral administration on indicators of the structural and functional state of the heart and heart rate variability (HRV) in RPIP has not yet been carried out.
The purpose of the study was to evaluate the effect of meldonium during its short-term parenteral use as part of combination therapy in RPIP in patients with chronic heart failure (CHF) on the clinical course of the recovery period, anti-ischemic and antianginal efficacy, structural and functional indicators and HRV.
Materials and methods
An open randomized study was conducted to study the effect of meldonium (idrinol, JSC Pharm, Russia) as part of combination therapy on the course of the recovery period in patients with RPIP. The study included 60 patients, men and women, aged 45 to 75 years at 3-4 weeks after MI with symptoms of heart failure of functional class II-III - FC (OSCH, 2002). After dividing the method of simple randomization into 2 groups, patients of the 1st (main) group ( n
=30) in addition to the basic therapy for IHD (enalapril, bisoprolol, acetylsalicylic acid, clopidogrel, spironolactone, torasemide, simvastatin, if necessary, calcium antagonists, nitrates), meldonium was prescribed at a dose of 1000 mg/day intravenously.
Patients of the 2nd (control) group ( n
= 30) received only basic therapy.
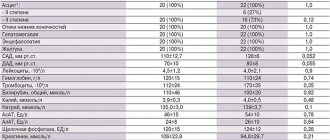
The duration of the study ranged from 10 to 14 days (average 12.4±0.8 days). Patients in the main and control groups were comparable in age, gender, severity of the disease, and the nature of the basic therapy performed. The average dosages of basic therapy drugs in groups 1 and 2 did not differ significantly. The initial characteristics of the patient groups are presented in Table.
1 .
To verify the FC of CHF, the criteria of OSCH (2002) and the 6-minute walk test (TST) were used. To diagnose and evaluate the effectiveness of CHF therapy, the level of N-terminal precursor of brain natriuretic peptide (NT-proBNP) in plasma was determined in all patients using an enzyme-linked immunosorbent assay (NT-proBNP determination kit, Biomedica, Slovakia) on a Liasis analyzer (Italy).
To establish episodes of cardiac arrhythmia and the frequency of ischemic episodes, 24-hour electrocardiogram (ECG) monitoring was carried out for 24 hours on a hardware-software complex with digital ECG recording Cardiotechnika-04 ZAO (INKART, Russia).
In all patients, the structural and functional parameters of the heart were studied using echocardiography on a Sonoline G50 device (Germany) in M-modal and two-dimensional modes in standard echocardiographic positions in accordance with recommendations for quantitative assessment of the structure and functions of the heart chambers [6]. To characterize the systolic function of the heart, the ejection fraction (EF) of the left ventricle (LV) was assessed according to Simpson (normal >45%). LV diastolic function was studied by assessing transmitral blood flow during the Valsalva maneuver and flow in the pulmonary veins. LV myocardial hypertrophy was diagnosed when the LV myocardial mass index (MMI) was greater than or equal to 125 g/m2 for men and 110 g/m2 for women. The types of LV remodeling were determined taking into account the relative wall thickness (RWT) of the LV and LVMI: a) concentric remodeling (CR): normal LV MMI and RWM >0.42; b) concentric hypertrophy (CH): increase in LV BMI and TVR >0.42; c) eccentric hypertrophy (EG): increase in LV IMM with normal TVR (<0.42).
HRV study was used to assess the functional state of the autonomic nervous system (ANS). ECG recording was performed on a VARIKARD-1.41 device (Russia) for 5 minutes in the morning at rest (15 minutes after the patient has adapted to the situation) in one of the standard leads in the supine position and during a 10-minute active orthostatic test [7] . The following HRV indicators were taken into account: SDNN (in ms) - standard deviation of RR interval values for the entire period under consideration; IN is the voltage index (in arbitrary units), TP (in ms2) is the total power of the HRV spectrum; HF, LF, VLF (in%) - respectively, the power of the spectrum of the high-frequency, low-frequency and very low-frequency components of variability in% of the total power of oscillations; IC (in arbitrary units) is a centralization index that reflects the degree of centralization of heart rhythm control.
Initial autonomic tone (VT) was assessed using a background test (lying down, at rest) by calculating the IN of regulatory systems. IN is the most important indicator characterizing the state of the central regulatory circuit; it is characterized by very high sensitivity to increased tone of the sympathetic part of the ANS (S-ANS) and increases several times under stress [8]. The direction of the initial VT and the nature of the sympathetic-parasympathetic relationship were assessed as follows: normotension - with CI from 30 to 90 arb. units, vagotonia - IN less than 30 arb. units, sympathicotonia with a moderate predominance of S-ANS tone - IN from 90 to 160 arb. units, hypersympathicotonia - SI more than 160 arb. units
Autonomic reactions that occur in response to external and internal stimuli characterize autonomic reactivity (VR). The functional reserves of autonomic regulation of cardiac activity can be assessed by an orthostatic test. Therefore, to assess the VR of both parts of the ANS, a test proposed by R.M. was performed. Baevsky [7], and BP was assessed using the ratio of the index of the index during the orthotest to the background index, taking into account its value. At the same time, normal, hyper- and asympathicotonic BP were distinguished [7].
In general, the initial VT and VR give an idea of the homeostatic capabilities of the body, and the vegetative support of activity gives an idea of its adaptive capabilities [8].
Statistical processing of data was carried out using the Statistica 7 program. To assess the significance of differences between groups, the t
Student's t test, sign test, angular transformation method (?).
Calculation of the significance of qualitative differences was assessed using Fisher's exact method. Differences were considered statistically significant at p
<0.05. The study protocol was approved by the Regional Ethics Committee.
results
In both groups, in patients who had suffered an MI, 10-14 days after the start of therapy, an improvement in the clinical condition and positive dynamics of the FC of CHF were revealed. There was a decrease in the level of CHF symptoms, more pronounced in the group of patients taking meldonium.
Both groups showed significant improvements in exercise tolerance assessed by TSH. Exercise tolerance according to TSH data in the group of patients receiving meldonium increased by 22.4% versus 17.9% in the control group ( p
>0.05).
There was a decrease in the FC of CHF, more pronounced in the 1st group of patients taking meldonium (by 9.3% compared to the 2nd group, where the same figure was 7.8%; p
> 0.05).
The positive effect of using meldonium for 10-14 days in addition to basic therapy on the course of CHF in RPIP was confirmed by a decrease in the level of NT-proBNP in patients of the main, 1st group by 18.7% (from 494.9±209.5 to 402. 4±124.5 pg/ml; p
>0.05), while in the control (2nd) group the level of NT-proBNP decreased by 4.8% (from 486.3±238.8 to 462.6± 206.3 pg/ml;
p
>0.05).
The difference between the final results in groups 1 and 2 were at the level of a trend ( p
<0.1).
The administration of meldonium in RPIP as part of combination therapy in patients with CHF at the end of 10-14 days of parenteral use was accompanied by a significant antianginal effect in the form of a significant reduction in angina attacks by 78.7% in the main group versus 20.3% in the control group and a decrease in the need for additional use of nitrates up to 1.2±0.12 nitrospray applications per week in the main group versus 1.83±0.19 in the control group ( p
<0,05).
In addition, we have established the antiarrhythmic and anti-ischemic effects of meldonium according to SM ECG data [9]. Thus, according to the results of the SM ECG, the use of meldonium as part of a combination treatment in the 1st group was accompanied by a significant decrease in the number of patients with episodes of heart rhythm disturbances (ventricular, atrial extrasystole, as well as short-term episodes of atrial fibrillation) compared to the 2nd (control) group: –84 and –17.6%, respectively. The differences between the groups in the final result were statistically significant. The number of patients with episodes of asymptomatic ischemia according to CM ECG data significantly decreased in group 1 with the addition of meldonium to basic therapy by -87.5% versus -42.9% in group 2 (control). Patients in both the main and control groups, due to the presence of episodes of arrhythmia and ischemia according to CM ECG data, underwent correction of basic therapy (increasing the dose of β-blockers).
It should be noted that the antianginal and anti-ischemic effects were not mediated by the effect of meldonium on hemodynamic parameters: the levels of SBP and DBP, as well as heart rate, did not undergo statistically significant changes during therapy.
A positive effect of meldonium as part of combination therapy on the structural and functional parameters of the heart in RPIP was noted (Table 2)
.
In patients of group 1, who received meldonium for 10-14 days, by the end of the study, EF increased by 5.2% ( p
>0.05), while in the control group - by 1.1%.
Initially, in group 1, DD was diagnosed in 93% of patients (see Table 2)
. During 10-14 days of meldonium use, the detection rate of DD decreased to 83%. In the 2nd (control) group, DD was also initially detected in 93% of patients, and by the end of the study the number of patients decreased to 87% (differences between groups in the final result were statistically insignificant).
Favorable changes during treatment with meldonium were also revealed in the geometry of the heart. A decrease in LV EDV was found in both groups, more significant with additional parenteral administration of meldonium (&Dgr; -3.6 and -1.6%; p
>0.05 in the main and control groups, respectively). LA sizes in patients of the 1st group decreased statistically insignificantly by 3.8%, in the 2nd group - by 3.1%.
Initially, all patients included in the study had all types of LV remodeling: CR, EG and CG. By the end of the study, in the 1st group receiving meldonium, there was a decrease in the number of patients with the most unfavorable types - EG and LV CG - from 90 to 83% and an increase in the number of patients with LV CR from 10 to 17% ( p
>0.05).
In the 2nd (control) group, there was a decrease in the number of patients with EG and LV CG from 87 to 83% and an increase in the number of patients with LV CR - from 13 to 17% ( p
> 0.05).
Of undoubted interest was the assessment of the effect of meldonium as part of combination therapy for CHF in RPIP with its short-term parenteral administration on HRV indicators reflecting the state of the ANS, since activation of the SAS plays an important role in the formation of LV remodeling in RPIP [3]. When studying HRV indicators initially in patients of both groups, a decrease in standard deviation (SDNN) <50 ms was revealed: 37.9 ± 2.4 ms in group 1 and 38.3 ± 2.27 ms in group 2. This indicates an increase in the activity of the S-ANS and is a prognostically unfavorable sign (indicates a high risk of dangerous arrhythmias and sudden coronary death) [7, 10]. It was found that in patients with CHF in the RPIP there was a pronounced centralization of heart rhythm control. This was reflected in an increase in the average values of IN (292.5±29.1 and 287.25±24.7 conventional units) and IC (6.93±0.8 and 6.61±0.73 units) in the main and control groups, respectively. During a 10-14-day use of meldonium in patients in RPIP as part of combination therapy, a vegetative-normalizing effect of the drug was noted. It manifested itself in the form of a significant increase in SDNN by 56.9% versus 20.6% in the control group, a statistically significant decrease in stress IC by 48.8% versus 11.3% in the control group, as well as a significant decrease in IC by 32.9% in the main group, while in the control group of patients this figure increased by 12.7% [9].
When assessing the initial VT of the cardiovascular system during the analysis of background IN according to HRV data, a state of sympathico- and hypersympathicotonia was revealed in patients of both groups at the beginning of the study (Fig. 1)
.
Figure 1. Dynamics of initial VT in patients with CHF in RPIP. Here and in Fig. 2: * — reliability of differences between the initial and final results; **- significance of differences between the percentages of groups 1 and 2 ( p
<0.05). However, against the background of the additional prescription of meldonium to the basic therapy in the 1st group of patients, favorable changes in the initial VT were noted in the form of a decrease in the number of cases of hypersympathicotonia and the appearance of patients with normotonia. In the 2nd (control) group, by the end of the study, the hypersympathicotonic type of initial VT remained predominant. The differences between the groups in the final result are statistically significant.
When analyzing the results of the test for BP, initially in both groups patients with a- and hypersympathicotonic BP predominated (Fig. 2)
.
Figure 2. Dynamics of RT in patients with CHF in RPIP. After 10-14 days of use of meldonium in RPIP as part of combination therapy in group 1, the normal type of BP significantly began to predominate in patients: the proportion of patients with hyper- and asympathicotonia decreased. In the 2nd (control) group, by the end of the study, the hypersympathicotonic type of VR became the most common.
Discussion
Meldonium is a drug from the group of partial inhibitors of free fatty acids (FFA); known primarily as a drug that has a non-hemodynamic anti-ischemic effect. Its antianginal effectiveness is most proven and in demand in clinical practice [11, 12], which was also noted in this study. The features of the pharmacological anti-ischemic effect of meldonium are mediated by a decrease in carnitine-dependent transport of fatty acids into the mitochondria of muscle tissue [13, 14], increased synthesis of γ-butyrobetaine and, as a consequence, induction of the formation of nitric oxide (NO) - one of the most effective natural agents that bind free radicals in the body. NO causes such effects of meldonium as a decrease in peripheral vascular resistance, a decrease in blood vessel spasm caused by norepinephrine or angiotensin II, inhibition of platelet aggregation and an increase in the elasticity of erythrocyte membranes. Meldonium has a selective effect specifically on the ischemic zone of various tissues, including the myocardium, with virtually no effect on areas not affected by ischemia (counteracting the steal effect) [15]. In addition, the anti-ischemic effect of meldonium can be mediated by the vegetative-normalizing effect of the drug, which was noted and described by us earlier [9]. The SM ECG data obtained during this study demonstrated a significant decrease in the number of patients with ischemic episodes in the main group taking meldonium, while in the control group, despite basic therapy, short-term episodes of ischemia continued to be recorded.
The antiarrhythmic effects of therapy identified by us with the prescription of meldonium for 10-14 days in patients in the RPIP can be explained by a decrease in the intracellular concentration of FFAs, which have a proarrhythmogenic property [16], as well as a decrease in membrane permeability for calcium ions and inhibition of the associated β-adrenergic response myocardium [17, 18]. In addition, the antiarrhythmogenic effect of meldonium may be mediated by the beneficial effect of the drug on the parameters of initial VT and BP, which was confirmed in the study by a statistically significant decrease in the number of patients with hypersympathicotonia and an increase in the number of patients with a normal type of BP. According to V.M. Mikhailov [10], favorable changes in HRV indicators are accompanied not only by a reduction in the risk of developing ventricular tachycardia and ventricular fibrillation, but also in the risk of sudden death in patients with MI.
Meldonium, when administered short-term parenterally into the RP in patients with CHF, has a beneficial effect on the structural and functional parameters of the heart and indicators of autonomic regulation, which can be of great clinical significance. The positive effect of meldonium on indicators of autonomic homeostasis of the heart according to the results of a HRV study may also be mediated by the ability to induce the formation of NO, since it is known that the implementation of the autonomic effect on the heart rhythm significantly depends on the adequate production of NO of neuronal origin [19]. According to the experimental work of E.V. Artyushkova et al. [20], using a model of L-NAME-induced NO deficiency, the ability of meldonium at a dose of 80 mg/kg was shown not only to increase the concentration of stable NO metabolites, but also to reduce the coefficient of endothelial dysfunction to the level in intact animals.
Conclusion
Intravenous use of meldonium for 10-14 days at a dose of 1000 mg/day as part of combination therapy in RPIP in patients with heart failure has a positive effect on the course of the recovery period: it reduces the manifestations of CHF, which is accompanied by an increase in TSH results, a decrease in the severity of the FC of CHF and a decrease level of NT-proBNP in the blood of patients; provides anti-ischemic and anti-arrhythmic effects, which improves the clinical condition of patients in the RPIP due to a significant reduction in the frequency of angina attacks and the need for additional intake of nitrates, reduces the number of episodes of arrhythmia and ischemia; has a beneficial effect on the structural and functional parameters of the heart (the number of patients with unfavorable types of LV remodeling decreases and the diastolic function of the heart improves); has a normalizing effect on the main indicators of HRV, increases the number of patients with normotension and significantly reduces the number of patients with hypersympathicotonic VT, which is accompanied by an increase in the number of patients with normal VR and a significant decrease in the number of patients with the hypersympathicotonic type of VR.
Mildronate
Active substance
Meldonium (as phosphate)
Mechanism of action
Pharmacological action: cardioprotective, antihypoxic, angioprotective, antianginal.
The drug is well absorbed by the gastrointestinal tract. Bioavailability – 78% in the first two hours after administration. Helps improve metabolism.
At increased loads, the drug Mildronate meets the oxygen needs of cells, has a tonic effect on them, protects them from damage and eliminates the accumulation of toxins.
During the use of the drug, the body quickly recovers energetically and acquires the ability to withstand high loads.
Mildronate is used to treat disorders of the blood supply to the brain, cardiovascular system and to improve physical and mental performance.
Mildronate is used to slow down the formation of necrotic zones and to shorten the rehabilitation period in acute ischemic damage to the myocardium.
In the treatment of heart failure to reduce the frequency of angina attacks, increase myocardial contractility, and increase tolerance to physical activity.
Used for fundus pathologies (vascular and dystrophic)
With withdrawal syndrome in the treatment of chronic alcoholism in order to eliminate functional disorders of the nervous system.
Indications for use
The drug is used as one of the components in the complex treatment of coronary heart disease (chronic heart failure, myocardial infarction, angina pectoris, dishormonal cardiopathy, etc.), disorders of the blood supply to the brain (chronic cerebral circulatory failure, cerebral strokes). Mildronate is also indicated for thrombosis of the central retinal vein and its branches, hemophthalmia and retinal hemorrhage of various etiologies, retinopathy of various etiologies (hypertensive, diabetic), physical activity (including athletes), decreased performance, withdrawal syndrome in chronic alcoholism ( as part of complex therapy).
Contraindications
Hypersensitivity to the components of the drug Mildronate. The drug is contraindicated in persons with increased intracranial pressure (with intracranial tumors, with impaired venous outflow). Mildronate should not be taken by children under 12 years of age. For more details, read the official instructions for the drug.
Side effects
In rare cases: itching, decreased blood pressure (hypotension), tachycardia, dyspeptic disorders.