A standard blood test necessarily includes an indicator of hemoglobin level. This analysis is called clinical and is the basis for the study of almost all diseases. The protein hemoglobin is found in blood cells - red blood cells. It performs an important function of cellular respiration. Thanks to the iron it contains, blood has a red color. The colorimetric principle for determining hemoglobin level is based precisely on this property of pigmentation. How to do such an analysis was suggested by the Swiss doctor Herman Sahli at the end of the 19th century.
Principle of implementation
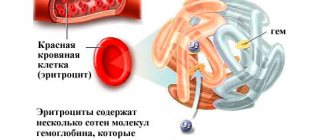
Determining the amount of hemoglobin using the Sali method is a classic method and is still used in many laboratories. Hemoglobin is a complex protein from the group of hemoproteins. It consists of two components: protein globin and non-protein heme. One hemoglobin molecule contains 4 hemes. At the center of each of them is an iron atom, which can reversibly combine with oxygen and carbon dioxide atoms without oxidizing. It is the presence of this element in the blood that gives it its characteristic color.
Principle of the study
The Sali method is based on a simple hemoglobin conversion reaction. To do this, a certain amount of hydrochloric acid is added to the blood product. As a result, a brown substance, chlorhematine, is formed in the test tube.
The content of converted hemoglobin can be determined by the color intensity of the resulting mixture. To do this, the solution is diluted with distilled water until its shade becomes identical to the substance in the control sealed test tube.
Important! Over time, the contents of the control preparations fade. As a result, there may be a deviation of the obtained data from real indicators by 1-3%.
What is a Sali hemometer?
To calculate hemoglobin levels, a device invented by a Swiss diagnostician is used. Sali's hemometer consists of three test tubes of the same size. Two test tubes are sealed and filled with a solution of blood in hydrochloric acid. This mixture is called hematin and has a brown tint. The intensity of the color depends on the amount of hemoglobin. In sealed hemometer tubes, the color corresponds to the standard hemoglobin content in the blood of a healthy person.
On a note! The normal level of hemoglobin in the blood of men is 130-160 g/l, in women – 120-140 g/l.
The third test tube has a scale on which gram percentages and hemometer units are marked. This container is filled with the analyzed material. Colorimetric analysis is performed visually by a laboratory assistant. The analyzed hematin is diluted with water to a certain color, which is compared with a standard sample.
Sali hemometer for determining hemoglobin level
To simplify laboratory analysis, the Sali hemometer was developed, which includes:
- tripod with 3 vertical sockets;
- 2 sealed test tubes with standard liquids;
- open graduated test tube;
- capillary (0.2 ml);
- water pipette;
- glass rod for stirring.
Methodology
To conduct a laboratory blood test using the Sali method, carry out the following steps. An empty hemometer tube is filled with hydrochloric acid to the bottom mark. The concentration of the solution should be 0.1 N. Next, 0.02 ml of the patient’s blood is drawn using a special capillary, which is included in the hemometer kit.
The capillary is immersed in an acidic solution and the material taken from the patient is carefully released into it. The blood should be under a layer of acid so that the acid itself remains transparent. Next, the contents of the test tube are mixed and kept untouched for 5-15 minutes. During this time, the interaction of liquids will occur, and brown hematin will be formed.
Distilled water is added drop by drop to the resulting substance until the color becomes identical to the color of the sample in the sealed hemometer tube. Each time the hematin is stirred with a glass rod. In this case, the liquid rises, its level corresponds to the scale of the control tube. As soon as the color of the analyzed material is equal to the standard color, the laboratory assistant notes which division of the gram-percentage scale corresponds to the rising liquid. To convert to the required grams per liter, the resulting figure is multiplied by 10.
Determination of hemoglobin content in blood using the Sali method
The blood contains an average of 14 g% hemoglobin, in women - 12 - 15, in men - 13 -16 g% (120-150 and 130-160 g/l, respectively). Determining the amount of hemoglobin is of great importance and is a mandatory component of a blood test. Hemoglobin is determined by colorimetric methods. Currently, the Sali method is rarely used in the clinic, since there are more accurate methods using photoelectrocolorimeters. Sali's hemometer is used for educational purposes.
Sali's method is based on the conversion of hemoglobin into brown hematin chloride by adding hydrochloric acid to the blood. The determination of hemoglobin is carried out using a colorimetric method, based on the following principle: if the test solution is brought to a color identical to the standard solution by dilution, then the concentration of dissolved substances in both solutions will be the same, and the amounts of substances will be correlated as their volumes. Knowing the amount of hemoglobin in the standard solution (16.7 g%, which is taken as 100% hemoglobin), it is easy to calculate its content in the test solution in relative percentages.
Sali's hemometer is a tripod, the back wall of which is made of frosted glass. Three test tubes of the same diameter are inserted into a rack. The two outer ones are sealed and contain a solution of hydrochloric acid hematin, the middle one is graduated and open. It is intended for the blood being tested. A capillary (marked 20 mm3), a glass rod and a pipette are attached to the device.
Purpose of the work: to determine the hemoglobin content in the blood using the Sali method.
Equipment: Sali hemometer, sterile scarifier, 0.1% HCI solution, filter paper, cotton wool, alcohol, ether, iodine, distilled water. The object of research is a person.
Carrying out work. HCI solution is poured into the middle tube of the hemometer to the bottom ring mark. Then blood is drawn from the finger in the usual way into the capillary up to the mark, removing the excess using filter paper. Blow blood onto the bottom of the middle test tube so that the top layer of hydrochloric acid remains uncolored. Without removing the pipette, rinse it with hydrochloric acid from the top layer.
After this, the contents of the test tube are mixed by hitting the bottom with a finger and left to stand for 8-10 minutes. This time is necessary for the complete conversion of hemoglobin into hematin hydrochloride. Then distilled water is added dropwise to the solution until the color of the resulting solution is the same as the standard color (adding water, mix the solution with a glass rod).
The number located at the level of the lower meniscus of the resulting solution shows the hemoglobin content in grams per 100 g of blood tested (when converted to SI units, the resulting figure is multiplied by 10, obtaining the hemoglobin value in grams per 1 liter of blood). You can also calculate the relative content of hemoglobin in the blood being tested.
Drawing up the protocol. Write down the hemoglobin content in the blood being tested. Calculate the relative percentage of hemoglobin as follows. Let's say the amount of hemoglobin in the blood corresponds to 14 hemometer divisions. Then, using the proportion, we calculate the relative hemoglobin content as a percentage. Compare the resulting value with the norm.
16.7 g% - 100%
14.0 g% - x
x =(100-14.0)/16.7
How accurate is the method for determining hemoglobin?
A Swiss diagnostician invented his own method for determining hemoglobin in blood at the end of the 19th century. At that time, this method was the most accurate. But new research, discoveries in the field of medicine and molecular diagnostics have created new methods. Hardware techniques have appeared that eliminate the human factor in research. They are able to evaluate biological material using more precise measurements.
Disadvantages of the Sali method
This method of determining the amount of hemoglobin in the blood is not widespread in the CIS countries. It has several serious disadvantages:
- Time. It takes at least 20 minutes to study the blood of 1 patient. At the same time, the use of automated systems allows you to get results in a few seconds.
- Subjectivity. Sali's chromatic technique is closely related to the individual perception of color. Accordingly, the results may vary even for 1 laboratory technician depending on external factors.
- Accuracy. The standard error of the method is 3 g/l. When using old samples of control tubes, high levels of bilirubin in the blood and poor lighting, the result of determining the hemoglobin level may differ even more, up to 30% from the actual values.
Veterinary and Animal Science
The hemiglobin cyanide method for determining blood hemoglobin has been approved as a unified method. Determination of hemoglobin using a Sali hemometer (GS-3) can be used only in the absence of a photoelectrocolorimeter, hemoglobinometer1 or spectrophotometer.
Sali's method.
Principle. Blood hemoglobin under the influence of 0.1 n. solution of hydrochloric acid turns into brown hematin chloride, its color intensity is compared with the standard.
Progress of determination. The tip of a clean and dry capillary from a hemometer is inserted into a drop of blood taken, located in the recess of the plate. Smoothly, without interruptions, using a rubber balloon, pump blood into the capillary to the circular mark (0.02 ml). The blood level in the capillary is adjusted by touching its tip with cotton wool or filter paper. Wipe the tip of the capillary from the blood and add it to 0.01 N. a solution of hydrochloric acid, located in a graduated test tube, releasing blood into the reagent in a thin stream, without stirring the liquid. Do not remove the capillary from the test tube; it is rinsed 2-3 times with a transparent supernatant 0.1 N. solution of hydrochloric acid, then removed from the liquid by touching its tip to the walls of the test tube, blow out the remaining liquid and remove it from the test tube. The blood and hydrochloric acid are immediately mixed by vigorously shaking the contents of the tube. Due to hemolysis of red blood cells and the formation of hematin chloride, the liquid turns brown and becomes transparent. The test tube from the hemometer, on which is marked a scale graduated in gram percentages, is inserted into the Sali hemometer, which has two standards, 5 minutes after displacement. After 5 minutes, distilled water is added to the test liquid in small portions, each time thoroughly mixing the liquid with a glass rod. The liquid draining from the stick should remain completely in the test tube. At the moment when the color of the liquid approaches the color of the standards, water begins to be added carefully, drop by drop. Dilution is completed as soon as the color of the test liquid matches the color of the standards. The color of the liquid standards is compared in daylight in transmitted light, held at arm's length at eye level. Determine which division of the scale corresponds to the lower meniscus of the liquid. The scale division value is 0.2 g%. When filling out the analysis form, the amount of hemoglobin is recorded in grams per 1 liter, for which the data obtained is multiplied by 10. After determining hemoglobin, the test tube and capillary are washed with distilled water, dried, and the hemometer is placed in a case.
Guide to practical classes on methods of clinical clinical laboratory research: Textbook. Manual - 4th ed., revised. And additionally - V.S. Ronin, G.M. Starobinets.-M.: Medicine, 1989.-320 p.